Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer's disease
- PMID: 18222096
- PMCID: PMC2720413
- DOI: 10.1016/j.neuroimage.2007.11.047
Automated ventricular mapping with multi-atlas fluid image alignment reveals genetic effects in Alzheimer's disease
Abstract
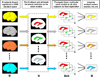
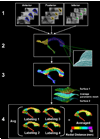
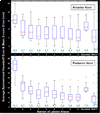
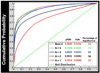
We developed and validated a new method to create automated 3D parametric surface models of the lateral ventricles in brain MRI scans, providing an efficient approach to monitor degenerative disease in clinical studies and drug trials. First, we used a set of parameterized surfaces to represent the ventricles in four subjects' manually labeled brain MRI scans (atlases). We fluidly registered each atlas and mesh model to MRIs from 17 Alzheimer's disease (AD) patients and 13 age- and gender-matched healthy elderly control subjects, and 18 asymptomatic ApoE4-carriers and 18 age- and gender-matched non-carriers. We examined genotyped healthy subjects with the goal of detecting subtle effects of a gene that confers heightened risk for Alzheimer's disease. We averaged the meshes extracted for each 3D MR data set, and combined the automated segmentations with a radial mapping approach to localize ventricular shape differences in patients. Validation experiments comparing automated and expert manual segmentations showed that (1) the Hausdorff labeling error rapidly decreased, and (2) the power to detect disease- and gene-related alterations improved, as the number of atlases, N, was increased from 1 to 9. In surface-based statistical maps, we detected more widespread and intense anatomical deficits as we increased the number of atlases. We formulated a statistical stopping criterion to determine the optimal number of atlases to use. Healthy ApoE4-carriers and those with AD showed local ventricular abnormalities. This high-throughput method for morphometric studies further motivates the combination of genetic and neuroimaging strategies in predicting AD progression and treatment response.
Figures



References
-
- Ashbumer J, Friston KJ. Spatial normalization. In: Toga AW, editor. Brain Warping, ch. 2. San Diego, USA: Academic Press; 1999. pp. 27–44.
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Ed 4. Washington, DC: American Psychiatric Association; 2000. (DSM-IV)
-
- Babalola KO, Cootes TF, Twining CJ, Petrovic V, Schestowitz R, Taylor CJ. Building 3D Statistical Shape Models using Groupwise Registration. Proceedings of the 12th annual meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006.
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc, Ser. B Method. 1995;57:289–300.
-
- Beyer K, Lao JI, Fernandez-Novoa L, Alvarez XA, Sellers MA, Cacabelos R. APOE epsilon 4 allele frequency in Alzheimer’s disease and vascular dementia in the Spanish population. Ann. N. Y. Acad. Sci. 1997;826:452–455. (1997 Sep 26) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

