ESCRT complexes and the biogenesis of multivesicular bodies
- PMID: 18222686
- PMCID: PMC2282067
- DOI: 10.1016/j.ceb.2007.12.002
ESCRT complexes and the biogenesis of multivesicular bodies
Abstract
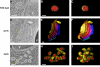
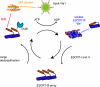
Multivesicular bodies (MVBs) are crucial intermediates in the trafficking of ubiquitinated receptors and other cargo destined for lysosomes. The formation of MVBs by invagination of the endosomal limiting membrane is catalyzed by the endosomal sorting complex required for transport (ESCRT) complexes, a process that has recently been visualized in three-dimensional detail by electron tomography. Structural and biochemical analysis of the upstream components, Vps27-Hse1, ESCRT-I, and ESCRT-II, shows how these complexes assemble and cluster cargo. Rapid progress has been made in understanding the assembly and disassembly of the ESCRT-III complex and the interactions of its subunits with MIT domain and other proteins. A key role for deubiquitination in the regulation of the system has been demonstrated. One central question remains largely unanswered, which is how the ESCRTs actually promote the invagination of the endosomal membrane.
Figures


References
-
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. - PubMed
-
- Russell MRG, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr. Opin. Cell Biol. 2006;18:422–428. - PubMed
-
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. - PubMed
-
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. ESCRT-III: An endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 2002;3:271–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

