Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression
- PMID: 18223090
- PMCID: PMC2293209
- DOI: 10.1128/JB.01736-07
Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression
Abstract
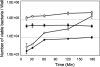
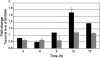
Campylobacter jejuni, a spiral-shaped gram-negative bacterium, is a leading bacterial cause of human food-borne illness. Acute disease is associated with C. jejuni invasion of the intestinal epithelium. Further, maximal host cell invasion requires the secretion of proteins termed Campylobacter invasion antigens (Cia). As bile acids are known to alter the pathogenic behavior of other gastrointestinal pathogens, we hypothesized that the virulence potential of Campylobacter may be triggered by the bile acid deoxycholate (DOC). In support of this hypothesis, culturing C. jejuni with a physiologically relevant concentration of DOC significantly altered the kinetics of cell invasion, as shown by gentamicin protection assays. In contrast to C. jejuni harvested from Mueller-Hinton (MH) agar plates, C. jejuni harvested from MH agar plates supplemented with DOC secreted the Cia proteins, as judged by metabolic labeling experiments. DOC was also found to induce the expression of the ciaB gene, as determined by beta-galactosidase reporter, real-time reverse transcription-PCR, and microarray analyses. Microarray analysis further revealed that DOC induced the expression of virulence genes (ciaB, cmeABC, dccR, and tlyA). In summary, we demonstrated that it is possible to enhance the pathogenic behavior of C. jejuni by modifying the culture conditions. These results provide a foundation for identifying genes expressed by C. jejuni in response to in vivo-like culture conditions.
Figures




Similar articles
-
Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal.J Infect Dis. 2001 Jun 1;183(11):1607-16. doi: 10.1086/320704. Epub 2001 May 1. J Infect Dis. 2001. PMID: 11343209
-
Presence of genes associated with adhesion, invasion, and toxin production in Campylobacter jejuni isolates and effect of temperature on their expression.Can J Microbiol. 2019 Apr;65(4):253-260. doi: 10.1139/cjm-2018-0539. Epub 2018 Dec 11. Can J Microbiol. 2019. PMID: 30532987
-
Virulence gene expression, adhesion and invasion of Campylobacter jejuni exposed to oxidative stress (H2O2).Int J Food Microbiol. 2016 Mar 2;220:33-8. doi: 10.1016/j.ijfoodmicro.2016.01.002. Epub 2016 Jan 7. Int J Food Microbiol. 2016. PMID: 26773256
-
Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms.Int J Med Microbiol. 2010 Apr;300(4):205-11. doi: 10.1016/j.ijmm.2009.07.002. Epub 2009 Aug 8. Int J Med Microbiol. 2010. PMID: 19665925 Review.
-
The pathogenesis of Campylobacter jejuni-mediated enteritis.Curr Issues Intest Microbiol. 2001 Sep;2(2):55-71. Curr Issues Intest Microbiol. 2001. PMID: 11721281 Review.
Cited by
-
Effect of environmental stress factors on the uptake and survival of Campylobacter jejuni in Acanthamoeba castellanii.BMC Microbiol. 2012 Oct 11;12:232. doi: 10.1186/1471-2180-12-232. BMC Microbiol. 2012. PMID: 23051891 Free PMC article.
-
Secondary Bile Acids and Tumorigenesis in Colorectal Cancer.Front Oncol. 2022 Apr 28;12:813745. doi: 10.3389/fonc.2022.813745. eCollection 2022. Front Oncol. 2022. PMID: 35574393 Free PMC article. Review.
-
Biochemical basis for activation of virulence genes by bile salts in Vibrio parahaemolyticus.Gut Microbes. 2017 Jul 4;8(4):366-373. doi: 10.1080/19490976.2017.1287655. Epub 2017 Jan 27. Gut Microbes. 2017. PMID: 28129014 Free PMC article. Review.
-
Metabolomic profiling uncovers diagnostic biomarkers and dysregulated pathways in Parkinson's disease.Front Neurol. 2025 Jun 4;16:1608031. doi: 10.3389/fneur.2025.1608031. eCollection 2025. Front Neurol. 2025. PMID: 40534749 Free PMC article.
-
Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis.Clin Dev Immunol. 2013;2013:526860. doi: 10.1155/2013/526860. Epub 2013 Nov 14. Clin Dev Immunol. 2013. PMID: 24324507 Free PMC article. Review.
References
-
- Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 321201-1206. - PubMed
-
- Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22703-714. - PubMed
-
- Begley, M., C. G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29625-651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

