Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference
- PMID: 18223117
- PMCID: PMC2268508
- DOI: 10.1128/EC.00316-07
Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference
Abstract
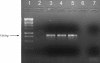
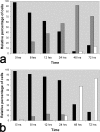
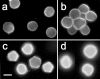
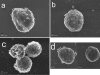
Acanthamoeba infections are difficult to treat due to often late diagnosis and the lack of effective and specific therapeutic agents. The most important reason for unsuccessful therapy seems to be the existence of a double-wall cyst stage that is highly resistant to the available treatments, causing reinfections. The major components of the Acanthamoeba cyst wall are acid-resistant proteins and cellulose. The latter has been reported to be the major component of the inner cyst wall. It has been demonstrated previously that glycogen is the main source of free glucose for the synthesis of cellulose in Acanthamoeba, partly as glycogen levels fall during the encystment process. In other lower eukaryotes (e.g., Dictyostelium discoideum), glycogen phosphorylase has been reported to be the main tool used for glycogen breakdown in order to maintain the free glucose levels during the encystment process. Therefore, it was hypothesized that the regulation of the key processes involved in the Acanthamoeba encystment may be similar to the previously reported regulation mechanisms in other lower eukaryotes. The catalytic domain of the glycogen phosphorylase was silenced using RNA interference methods, and the effect of this phenomenon was assessed by light and electron microscopy analyses, calcofluor staining, expression zymogram assays, and Northern and Western blot analyses of both small interfering RNA-treated and control cells. The present report establishes the role of glycogen phosphorylase during the encystment process of Acanthamoeba. Moreover, the obtained results demonstrate that the enzyme is required for cyst wall assembly, mainly for the formation of the cell wall inner layer.
Figures




References
-
- Anderson, I. J., R. F. Watkins, J. Samuelson, D. F. Spencer, W. H. Majoros, M. W. Gray, and B. J. Loftus. 2005. Gene discovery in the Acanthamoeba castellanii genome. Protist 156203-214. - PubMed
-
- Bishop, J. D., B. C. Moon, F. Harrow, D. Ratner, R. H. Gomer, R. P. Dottin, and D. T. Brazill. 2002. A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J. Biol. Chem. 3632430-32437. - PubMed
-
- Blanton, W. E., and C. L. Villemez. 1978. Molecular-size and chain-length distribution in Acanthamoeba cellulose. J. Protozool. 25264-267.
-
- Booton, G. C., D. J. Kelly, Y. W. Chu, D. V. Seal, E. Houang, D. S. Lam, T. J. Byers, and P. A. Fuerst. 2002. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 401621-1625. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

