Arabidopsis NAD-malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism
- PMID: 18223148
- PMCID: PMC2287332
- DOI: 10.1104/pp.107.114975
Arabidopsis NAD-malic enzyme functions as a homodimer and heterodimer and has a major impact on nocturnal metabolism
Abstract
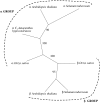
Although the nonphotosynthetic NAD-malic enzyme (NAD-ME) was assumed to play a central role in the metabolite flux through the tricarboxylic acid cycle, the knowledge on this enzyme is still limited. Here, we report on the identification and characterization of two genes encoding mitochondrial NAD-MEs from Arabidopsis (Arabidopsis thaliana), AtNAD-ME1 and AtNAD-ME2. The encoded proteins can be grouped into the two clades found in the plant NAD-ME phylogenetic tree. AtNAD-ME1 belongs to the clade that includes known alpha-subunits with molecular masses of approximately 65 kD, while AtNAD-ME2 clusters with the known beta-subunits with molecular masses of approximately 58 kD. The separated recombinant proteins showed NAD-ME activity, presented comparable kinetic properties, and are dimers in their active conformation. Native electrophoresis coupled to denaturing electrophoresis revealed that in vivo AtNAD-ME forms a dimer of nonidentical subunits in Arabidopsis. Further support for this conclusion was obtained by reconstitution of the active heterodimer in vitro. The characterization of loss-of-function mutants for both AtNAD-MEs indicated that both proteins also exhibit enzymatic activity in vivo. Neither the single nor the double mutants showed a growth or developmental phenotype, suggesting that NAD-ME activity is not essential for normal autotrophic development. Nevertheless, metabolic profiling of plants completely lacking NAD-ME activity revealed differential patterns of modifications in light and dark periods and indicates a major role for NAD-MEs during nocturnal metabolism.
Figures




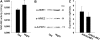

Similar articles
-
Three different and tissue-specific NAD-malic enzymes generated by alternative subunit association in Arabidopsis thaliana.J Biol Chem. 2010 Apr 16;285(16):11870-9. doi: 10.1074/jbc.M109.097477. Epub 2010 Feb 4. J Biol Chem. 2010. PMID: 20133948 Free PMC article.
-
NAD-malic enzymes of Arabidopsis thaliana display distinct kinetic mechanisms that support differences in physiological control.Biochem J. 2010 Sep 1;430(2):295-303. doi: 10.1042/BJ20100497. Biochem J. 2010. PMID: 20528775
-
Specific Arabidopsis thaliana malic enzyme isoforms can provide anaplerotic pyruvate carboxylation function in Saccharomyces cerevisiae.FEBS J. 2017 Feb;284(4):654-665. doi: 10.1111/febs.14013. Epub 2017 Feb 1. FEBS J. 2017. PMID: 28075062
-
Malate decarboxylases: evolution and roles of NAD(P)-ME isoforms in species performing C(4) and C(3) photosynthesis.J Exp Bot. 2011 May;62(9):3061-9. doi: 10.1093/jxb/err024. Epub 2011 Mar 31. J Exp Bot. 2011. PMID: 21459769 Review.
-
More to NAD+ than meets the eye: A regulator of metabolic pools and gene expression in Arabidopsis.Free Radic Biol Med. 2018 Jul;122:86-95. doi: 10.1016/j.freeradbiomed.2018.01.003. Epub 2018 Jan 5. Free Radic Biol Med. 2018. PMID: 29309893 Review.
Cited by
-
Analysis of Arabidopsis with highly reduced levels of malate and fumarate sheds light on the role of these organic acids as storage carbon molecules.Plant Physiol. 2010 Mar;152(3):1251-62. doi: 10.1104/pp.109.151795. Epub 2010 Jan 27. Plant Physiol. 2010. PMID: 20107023 Free PMC article.
-
Inferred Subcellular Localization of Peroxisomal Matrix Proteins of Guillardia theta Suggests an Important Role of Peroxisomes in Cryptophytes.Front Plant Sci. 2022 Jun 16;13:889662. doi: 10.3389/fpls.2022.889662. eCollection 2022. Front Plant Sci. 2022. PMID: 35783940 Free PMC article.
-
2-Hydroxy Acids in Plant Metabolism.Arabidopsis Book. 2015 Sep 4;13:e0182. doi: 10.1199/tab.0182. eCollection 2015. Arabidopsis Book. 2015. PMID: 26380567 Free PMC article.
-
Cucumber malate decarboxylase, CsNADP-ME2, functions in the balance of carbon and amino acid metabolism in fruit.Hortic Res. 2023 Oct 25;10(12):uhad216. doi: 10.1093/hr/uhad216. eCollection 2023 Dec. Hortic Res. 2023. PMID: 38077499 Free PMC article.
-
The mitochondrial pyruvate carrier (MPC) complex mediates one of three pyruvate-supplying pathways that sustain Arabidopsis respiratory metabolism.Plant Cell. 2021 Aug 31;33(8):2776-2793. doi: 10.1093/plcell/koab148. Plant Cell. 2021. PMID: 34137858 Free PMC article.
References
-
- Agius SC, Rasmusson AG, Moller IM (2001) NAD(P) turnover in plant mitochondria. Aust J Plant Physiol 28 461–470
-
- Allen BL, Harris BG (1981) Purification of malic enzyme from Ascaris suum using NAD+-agarose. Mol Biochem Parasitol 2 367–372 - PubMed
-
- Artus NN, Edwards GE (1985) Properties of leaf NAD-malic enzyme from the inducible crassulacean acid metabolism species Mesembryanthemum crystallinum. Plant Cell Physiol 26 341–350
-
- Burnell JN (1987) Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 species: properties of NAD-malic enzyme from Urochloa panicoides. Aust J Plant Physiol 14 517–525
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

