B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells
- PMID: 18223165
- PMCID: PMC2275025
- DOI: 10.1182/blood-2007-11-123141
B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells
Abstract
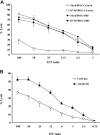
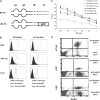
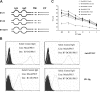
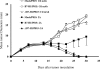
B7-H1 is an immunoglobulin-like immune suppressive molecule broadly detectable on the majority of human and rodent cancers, and its functions have been attributed to delivering an inhibitory signal to its counter-receptor programmed death-1 (PD-1) on T cells. Here we report that B7-H1 on cancer cells receives a signal from PD-1 to rapidly induce resistance against T cell-mediated killing because crippling signaling capacity of B7-H1 but not PD-1 ablates this resistance. Importantly, loss of B7-H1 signaling is accompanied by increased susceptibility to immune-mediated tumoricidal activity. In addition to resistance against T-cell destruction, B7-H1+ cancer cells also become refractory to apoptosis induced by Fas ligation or the protein kinase inhibitor Staurosporine. Our study reveals a new mechanism by which cancer cells use a receptor on immune cells as a ligand to induce resistance to therapy.
Figures

References
-
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. - PubMed
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. - PubMed
-
- Garrido F, Cabrera T, Concha A, et al. Natural history of HLA expression during tumour development. Immunol Today. 1993;14:491–499. - PubMed
-
- Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. - PubMed
-
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

