Small-molecule aggregates inhibit amyloid polymerization
- PMID: 18223646
- PMCID: PMC2730835
- DOI: 10.1038/nchembio.65
Small-molecule aggregates inhibit amyloid polymerization
Abstract
Many amyloid inhibitors resemble molecules that form chemical aggregates, which are known to inhibit many proteins. Eight known chemical aggregators inhibited amyloid formation of the yeast and mouse prion proteins Sup35 and recMoPrP in a manner characteristic of colloidal inhibition. Similarly, three known anti-amyloid molecules inhibited beta-lactamase in a detergent-dependent manner, which suggests that they too form colloidal aggregates. The colloids localized to preformed fibers and prevented new fiber formation in electron micrographs. They also blocked infection of yeast cells with Sup35 prions, which suggests that colloidal inhibition may be relevant in more biological milieus.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures
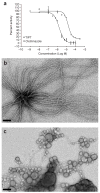
Comment in
-
Aggregator compounds confound amyloid fibrillization assay.Nat Chem Biol. 2008 Mar;4(3):159-60. doi: 10.1038/nchembio0308-159. Nat Chem Biol. 2008. PMID: 18277973 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/46499856
- PubChem-Substance/46499857
- PubChem-Substance/46499858
- PubChem-Substance/46499859
- PubChem-Substance/46499860
- PubChem-Substance/46499861
- PubChem-Substance/46499862
- PubChem-Substance/46499863
- PubChem-Substance/46499864
- PubChem-Substance/46499865
- PubChem-Substance/46499866
- PubChem-Substance/46499867
- PubChem-Substance/46499868
- PubChem-Substance/46499869
- PubChem-Substance/46499870
- PubChem-Substance/46499871
- PubChem-Substance/46499872
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

