Comparative study on transduction and toxicity of protein transduction domains
- PMID: 18223668
- PMCID: PMC2275450
- DOI: 10.1038/sj.bjp.0707678
Comparative study on transduction and toxicity of protein transduction domains
Abstract
Background and purpose: Protein transduction domains (PTDs), such as Tat, antennapedia homeoprotein (Antp), Rev and VP22, have been extensively utilized for intracellular delivery of biologically active macromolecules in vitro and in vivo. There is little known, however, about the relative transduction efficacy, cytotoxicity and internalization mechanism of individual PTDs.
Experimental approach: We examined the cargo delivery efficacies of four major PTDs (Tat, Antp, Rev and VP22) and evaluated their toxicities and cell internalizing pathways in various cell lines.
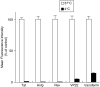
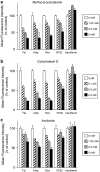
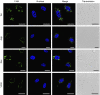
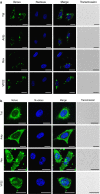
Key results: The relative order of the transduction efficacy of these PTDs conjugated to fluorescein was Rev>Antp>Tat>VP22, independent of cell type (HeLa, HaCaT, A431, Jurkat, MOLT-4 and HL60 cells). Antp produced significant toxicity in HeLa and Jurkat cells, and Rev produced significant toxicity in Jurkat cells. Flow cytometric analysis demonstrated that the uptake of PTD-fluorescein conjugate was dose-dependently inhibited by methyl-beta-cyclodextrin, cytochalasin D and amiloride, indicating that all four PTDs were internalized by the macropinocytotic pathway. Accordingly, in cells co-treated with 'Tat-fused' endosome-disruptive HA2 peptides (HA2-Tat) and independent PTD-fluorescent protein conjugates, fluorescence spread throughout the cytosol, indicating that all four PTDs were internalized into the same vesicles as Tat.
Conclusions and implications: These findings suggest that macropinocytosis-dependent internalization is a crucial step in PTD-mediated molecular transduction. From the viewpoint of developing effective and safe protein transduction technology, although Tat was the most versatile carrier among the peptides studied, PTDs should be selected based on their individual characteristics.
Figures



Similar articles
-
Antennapedia and HIV transactivator of transcription (TAT) "protein transduction domains" promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans.J Biol Chem. 2003 Sep 12;278(37):35109-14. doi: 10.1074/jbc.M301726200. Epub 2003 Jun 30. J Biol Chem. 2003. PMID: 12837762
-
Protein transduction domains fused to virus receptors improve cellular virus uptake and enhance oncolysis by tumor-specific replicating vectors.J Virol. 2004 Dec;78(24):13743-54. doi: 10.1128/JVI.78.24.13743-13754.2004. J Virol. 2004. PMID: 15564483 Free PMC article.
-
Protein transport in human cells mediated by covalently and noncovalently conjugated arginine-rich intracellular delivery peptides.Peptides. 2009 Sep;30(9):1669-78. doi: 10.1016/j.peptides.2009.06.006. Epub 2009 Jun 12. Peptides. 2009. PMID: 19524630
-
Intracellular transduction and potential of Tat PTD and its analogs: from basic drug delivery mechanism to application.Expert Opin Drug Deliv. 2012 Apr;9(4):457-72. doi: 10.1517/17425247.2012.663351. Expert Opin Drug Deliv. 2012. PMID: 22432469 Review.
-
Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides.Adv Drug Deliv Rev. 2005 Feb 28;57(4):637-51. doi: 10.1016/j.addr.2004.10.007. Epub 2004 Dec 22. Adv Drug Deliv Rev. 2005. PMID: 15722168 Review.
Cited by
-
Synergistic Enhancement of Antitumor Efficacy by PEGylated Multi-walled Carbon Nanotubes Modified with Cell-Penetrating Peptide TAT.Nanoscale Res Lett. 2016 Dec;11(1):452. doi: 10.1186/s11671-016-1672-6. Epub 2016 Oct 10. Nanoscale Res Lett. 2016. PMID: 27726120 Free PMC article.
-
Efficient protein transduction method using cationic peptides and lipids.J Biomed Biotechnol. 2011;2011:872065. doi: 10.1155/2011/872065. Epub 2011 Oct 9. J Biomed Biotechnol. 2011. PMID: 22007148 Free PMC article.
-
TAT fusion protein transduction into isolated mitochondria is accelerated by sodium channel inhibitors.Biochemistry. 2010 Nov 9;49(44):9470-9. doi: 10.1021/bi101057v. Biochemistry. 2010. PMID: 20925426 Free PMC article.
-
Transduction of the MPG-tagged fusion protein into mammalian cells and oocytes depends on amiloride-sensitive endocytic pathway.BMC Biotechnol. 2009 Aug 26;9:73. doi: 10.1186/1472-6750-9-73. BMC Biotechnol. 2009. PMID: 19706197 Free PMC article.
-
The interaction of mycobacterial protein Rv2966c with host chromatin is mediated through non-CpG methylation and histone H3/H4 binding.Nucleic Acids Res. 2015 Apr 30;43(8):3922-37. doi: 10.1093/nar/gkv261. Epub 2015 Mar 30. Nucleic Acids Res. 2015. PMID: 25824946 Free PMC article.
References
-
- Berlose JP, Convert O, Derossi D, Brunissen A, Chassaing G. Conformational and associative behaviours of the third helix of antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996;242:372–386. - PubMed
-
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. - PubMed
-
- Brusic V, Marina O, Wu CJ, Reinherz EL. Proteome informatics for cancer research: from molecules to clinic. Proteomics. 2007;7:976–991. - PubMed
-
- Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) ‘protein transduction domains' promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–35114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

