Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention?
- PMID: 18223674
- PMCID: PMC2268072
- DOI: 10.1038/sj.bjp.0707603
Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention?
Abstract
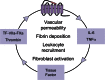
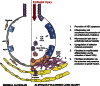
There is compelling evidence that uncontrolled activation of the coagulation cascade following lung injury contributes to the development of lung inflammation and fibrosis in acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and fibrotic lung disease. This article reviews our current understanding of the mechanisms leading to the activation of the coagulation cascade in response to lung injury and the evidence that excessive procoagulant activity is of pathophysiological significance in these disease settings. Current evidence suggests that the tissue factor-dependent extrinsic pathway is the predominant mechanism by which the coagulation cascade is locally activated in the lungs of patients with ALI/ARDS and pulmonary fibrosis. Whilst, fibrin deposition might contribute to the pathophysiology of ALI/ARDS following systemic insult; current evidence suggests that the cellular effects mediated via activation of proteinase-activated receptors (PARs) may be of particular importance in influencing inflammatory and fibroproliferative responses in experimental models involving direct injury to the lung. In this regard, studies in PAR(1) knockout mice have shown that this receptor plays a major role in orchestrating the interplay between coagulation, inflammation and lung fibrosis. This review will focus on our current understanding of excessive procoagulant signalling in acute and chronic lung injury and will highlight the novel opportunities that this may present for therapeutic intervention.
Figures


References
-
- Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999;21:693–700. - PubMed
-
- Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, et al. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–3585. - PubMed
-
- Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EG, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost. 2003;1:1935–1944. - PubMed
-
- Barry GD, Le GT, Fairlie DP. Agonists and antagonists of protease activated receptors (PARs) Curr Med Chem. 2006;13:243–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

