Testosterone depot injection in male hypogonadism: a critical appraisal
- PMID: 18225458
- PMCID: PMC2686335
Testosterone depot injection in male hypogonadism: a critical appraisal
Abstract
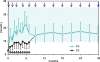
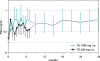
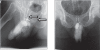
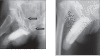
Testosterone compounds have been available for almost 70 years, but the pharmaceutical formulations have been less than ideal. Traditionally, injectable testosterone esters have been used for treatment, but they generate supranormal testosterone levels shortly after the 2- to 3-weekly injection interval and then testosterone levels decline very rapidly, becoming subnormal in the days before the next injection. The rapid fluctuations in plasma testosterone are subjectively experienced as disagreeable. Testosterone undecanoate is a new injectable testosterone preparation with a considerably better pharmacokinetic profile. After 2 initial injections with a 6-week interval, the following intervals between two injections are almost always 12-weeks, amounting eventually to a total of 4 injections per year. Plasma testosterone levels with this preparation are nearly always in the range of normal men, so are its metabolic products estradiol and dihydrotestosterone. The "roller coaster" effects of traditional parenteral testosterone injections are not apparent. It reverses the effects of hypogonadism on bone and muscle and metabolic parameters and on sexual functions. Its safety profile is excellent due to the continuous normalcy of plasma testosterone levels. No polycythemia has been observed, and no adverse effects on lipid profiles. Prostate safety parameters are well within reference limits. There was no impairment of uroflow. Testosterone undecanoate is a valuable contribution to the treatment options of androgen deficiency.
Figures

References
-
- Anderson RA, et al. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5 alpha-reductase activity in oligozoospermic men administered supraphysiological doses of testosterone. J Clin Endocrinol Metab. 1996;81:902–8. - PubMed
-
- Bagatell CJ, Bremner WJ. Androgen and progestagen effects on plasma lipids. Prog Cardiovasc Dis. 1995;38:255–71. - PubMed
-
- Bagchus WM, et al. Important effect of food on the bioavailability of oral testosterone undecanoate. Pharmacotherapy. 2003;23:319–25. - PubMed
-
- Behre HM, et al. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 1994;40:341–9. - PubMed
-
- Behre HM, et al. Pharmacology of testosterone preparations. In: Nieschlag E, Behre HM, editors. Testosterone, action, deficiency, substitution. Cambridge University Press; 2004. pp. 405–44.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

