Analysis of flavin oxidation and electron-transfer inhibition in Plasmodium falciparum dihydroorotate dehydrogenase
- PMID: 18225919
- PMCID: PMC2868338
- DOI: 10.1021/bi702218c
Analysis of flavin oxidation and electron-transfer inhibition in Plasmodium falciparum dihydroorotate dehydrogenase
Abstract
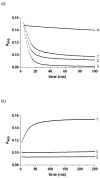
Plasmodium falciparum dihydroorotate dehydrogenase (pfDHODH) is a flavin-dependent mitochondrial enzyme that provides the only route to pyrimidine biosynthesis in the parasite. Clinically significant inhibitors of human DHODH (e.g., A77 1726) bind to a pocket on the opposite face of the flavin cofactor from dihydroorotate (DHO). This pocket demonstrates considerable sequence variability, which has allowed species-specific inhibitors of the malarial enzyme to be identified. Ubiquinone (CoQ), the physiological oxidant in the reaction, has been postulated to bind this site despite a lack of structural evidence. To more clearly define the residues involved in CoQ binding and catalysis, we undertook site-directed mutagenesis of seven residues in the structurally defined A77 1726 binding site, which we term the species-selective inhibitor site. Mutation of several of these residues (H185, F188, and F227) to Ala substantially decreased the affinity of pfDHODH-specific inhibitors (40-240-fold). In contrast, only a modest increase in the Kmapp for CoQ was observed, although mutation of Y528 in particular caused a substantial reduction in kcat (40-100-fold decrease). Pre-steady-state kinetic analysis by single wavelength stopped-flow spectroscopy showed that the mutations had no effect on the rate of the DHO-dependent reductive half-reaction, but most reduced the rate of the CoQ-dependent flavin oxidation step (3-20-fold decrease), while not significantly altering the Kdox for CoQ. As with the mutants, inhibitors that bind this site block the CoQ-dependent oxidative half-reaction without affecting the DHO-dependent step. These results identify residues involved in inhibitor binding and electron transfer to CoQ. Importantly, the data provide compelling evidence that the binding sites for CoQ and species-selective site inhibitors do not overlap, and they suggest instead that inhibitors act either by blocking the electron path between flavin and CoQ or by stabilizing a conformation that excludes CoQ binding.
Figures








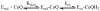
Similar articles
-
Malarial dihydroorotate dehydrogenase. Substrate and inhibitor specificity.J Biol Chem. 2002 Nov 1;277(44):41827-34. doi: 10.1074/jbc.M206854200. Epub 2002 Aug 19. J Biol Chem. 2002. PMID: 12189151
-
High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase.J Biol Chem. 2005 Jun 10;280(23):21847-53. doi: 10.1074/jbc.M501100200. Epub 2005 Mar 28. J Biol Chem. 2005. PMID: 15795226
-
Structure of Plasmodium falciparum dihydroorotate dehydrogenase with a bound inhibitor.Acta Crystallogr D Biol Crystallogr. 2006 Mar;62(Pt 3):312-23. doi: 10.1107/S0907444905042642. Epub 2006 Feb 22. Acta Crystallogr D Biol Crystallogr. 2006. PMID: 16510978
-
Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors.Mini Rev Med Chem. 2011 Oct;11(12):1039-55. doi: 10.2174/138955711797247707. Mini Rev Med Chem. 2011. PMID: 21861807 Review.
-
Recent advances on patents of Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) inhibitors as antimalarial agents.Expert Opin Ther Pat. 2023 Jul-Dec;33(9):579-596. doi: 10.1080/13543776.2023.2280596. Epub 2023 Nov 28. Expert Opin Ther Pat. 2023. PMID: 37942637 Review.
Cited by
-
Open-source discovery of chemical leads for next-generation chemoprotective antimalarials.Science. 2018 Dec 7;362(6419):eaat9446. doi: 10.1126/science.aat9446. Science. 2018. PMID: 30523084 Free PMC article.
-
Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum.J Med Chem. 2008 Jun 26;51(12):3649-53. doi: 10.1021/jm8001026. Epub 2008 Jun 4. J Med Chem. 2008. PMID: 18522386 Free PMC article.
-
Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds.J Biol Chem. 2009 Sep 25;284(39):26999-7009. doi: 10.1074/jbc.M109.028589. Epub 2009 Jul 28. J Biol Chem. 2009. PMID: 19640844 Free PMC article.
-
Identification of 3,4-Dihydro-2H,6H-pyrimido[1,2-c][1,3]benzothiazin-6-imine Derivatives as Novel Selective Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase.Int J Mol Sci. 2021 Jul 5;22(13):7236. doi: 10.3390/ijms22137236. Int J Mol Sci. 2021. PMID: 34281290 Free PMC article.
-
The X-ray structure of Plasmodium falciparum dihydroorotate dehydrogenase bound to a potent and selective N-phenylbenzamide inhibitor reveals novel binding-site interactions.Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):553-9. doi: 10.1107/S2053230X15000989. Epub 2015 Apr 21. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945708 Free PMC article.
References
-
- Nara T, Hshimoto T, Aoki T. Evolutionary implications of the mosaic pyrimidine-biosynthetic pathway in eukaryotes. Gene. 2000;257:209–222. - PubMed
-
- Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. - PubMed
-
- Bjornberg O, Jordan DB, Palfey BA, Jensen KF. Dihydrooxonate is a substrate of dihydroorotate dehydrogenase (DHOD) providing evidence for involvement of cysteine and serine residues in base catalysis. Arch Biochem Biophys. 2001;391:286–294. - PubMed
-
- Marcinkeviciene J, Tinney LM, Wang KH, Rogers MJ, Copeland RA. Dihydroorotate dehydrogenase B of Enterococcus faecalis. Characterization and insights into chemical mechanism. Biochemistry. 1999;38:13129–13137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

