Nuc2p, a subunit of the anaphase-promoting complex, inhibits septation initiation network following cytokinesis in fission yeast
- PMID: 18225957
- PMCID: PMC2213707
- DOI: 10.1371/journal.pgen.0040017
Nuc2p, a subunit of the anaphase-promoting complex, inhibits septation initiation network following cytokinesis in fission yeast
Abstract
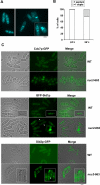
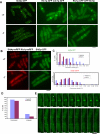
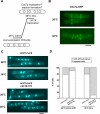
In most cell types, mitosis and cytokinesis are tightly coupled such that cytokinesis occurs only once per cell cycle. The fission yeast Schizosaccharomyces pombe divides using an actomyosin-based contractile ring and is an attractive model for the study of the links between mitosis and cytokinesis. In fission yeast, the anaphase-promoting complex/cyclosome (APC/C) and the septation initiation network (SIN), a spindle pole body (SPB)-associated GTPase-driven signaling cascade, function sequentially to ensure proper coordination of mitosis and cytokinesis. Here, we find a novel interplay between the tetratricopeptide repeat (TPR) domain-containing subunit of the APC/C, Nuc2p, and the SIN, that appears to not involve other subunits of the APC/C. Overproduction of Nuc2p led to an increase in the presence of multinucleated cells, which correlated with a defect in actomyosin ring maintenance and localization of the SIN component protein kinases Cdc7p and Sid1p to the SPBs, indicative of defective SIN signaling. Conversely, loss of Nuc2p function led to increased SIN signaling, characterized by the persistent localization of Cdc7p and Sid1p on SPBs and assembly of multiple actomyosin rings and division septa. Nuc2p appears to function independently of the checkpoint with FHA and ring finger (CHFR)-related protein Dma1p, a known inhibitor of the SIN in fission yeast. Genetic and biochemical analyses established that Nuc2p might influence the nucleotide state of Spg1p GTPase, a key regulator of the SIN. We propose that Nuc2p, by inhibiting the SIN after cell division, prevents further deleterious cytokinetic events, thereby contributing to genome stability.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures





Similar articles
-
SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast.Curr Biol. 2011 Dec 6;21(23):1968-78. doi: 10.1016/j.cub.2011.10.051. Epub 2011 Nov 23. Curr Biol. 2011. PMID: 22119525 Free PMC article.
-
Cytokinetic actomyosin ring formation and septation in fission yeast are dependent on the full recruitment of the polo-like kinase Plo1 to the spindle pole body and a functional spindle assembly checkpoint.J Cell Sci. 2002 Sep 15;115(Pt 18):3575-86. doi: 10.1242/jcs.00031. J Cell Sci. 2002. PMID: 12186944
-
Relief of the Dma1-mediated checkpoint requires Dma1 autoubiquitination and dynamic localization.Mol Biol Cell. 2018 Sep 1;29(18):2176-2189. doi: 10.1091/mbc.E18-04-0261. Epub 2018 Jul 5. Mol Biol Cell. 2018. PMID: 29975113 Free PMC article.
-
Polar opposites: Fine-tuning cytokinesis through SIN asymmetry.Cytoskeleton (Hoboken). 2012 Oct;69(10):686-99. doi: 10.1002/cm.21044. Epub 2012 Jul 11. Cytoskeleton (Hoboken). 2012. PMID: 22786806 Free PMC article. Review.
-
Pombe's thirteen - control of fission yeast cell division by the septation initiation network.J Cell Sci. 2015 Apr 15;128(8):1465-74. doi: 10.1242/jcs.094821. Epub 2015 Feb 17. J Cell Sci. 2015. PMID: 25690009 Review.
Cited by
-
Impaired coenzyme A synthesis in fission yeast causes defective mitosis, quiescence-exit failure, histone hypoacetylation and fragile DNA.Open Biol. 2012 Sep;2(9):120117. doi: 10.1098/rsob.120117. Open Biol. 2012. PMID: 23091701 Free PMC article.
-
An Extended, Boolean Model of the Septation Initiation Network in S.Pombe Provides Insights into Its Regulation.PLoS One. 2015 Aug 5;10(8):e0134214. doi: 10.1371/journal.pone.0134214. eCollection 2015. PLoS One. 2015. PMID: 26244885 Free PMC article.
-
Dividing the spoils of growth and the cell cycle: The fission yeast as a model for the study of cytokinesis.Cytoskeleton (Hoboken). 2011 Feb;68(2):69-88. doi: 10.1002/cm.20500. Cytoskeleton (Hoboken). 2011. PMID: 21246752 Free PMC article. Review.
-
Binding partner switching on microtubules and aurora-B in the mitosis to cytokinesis transition.Mol Cell Proteomics. 2010 Feb;9(2):336-50. doi: 10.1074/mcp.M900308-MCP200. Epub 2009 Sep 28. Mol Cell Proteomics. 2010. PMID: 19786723 Free PMC article.
References
-
- Hartwell LH, Weinert TA. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. - PubMed
-
- Romanowski P, Madine MA. Mechanisms restricting DNA replication to once per cell cycle: MCMS, pre-replicative complexes and kinases. Trends Cell Biol. 1996;6:184–188. - PubMed
-
- McCollum D, Gould KL. Timing is everything: Regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11:89–95. - PubMed
-
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe . Nature. 1992;360:84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

