Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model
- PMID: 18226258
- PMCID: PMC2266897
- DOI: 10.1186/1471-5945-8-1
Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model
Abstract
Background: Skin manifestations of Tuberous Sclerosis Complex (TSC) cause significant morbidity. The molecular mechanism underlying TSC is understood and there is evidence that systemic treatment with rapamycin or other mTOR inhibitors may be a useful approach to targeted therapy for the kidney and brain manifestations. Here we investigate topical rapamycin in a mouse model for TSC-related tumors.
Methods: 0.4% and 0.8% rapamycin ointments were applied to nude mice bearing subcutaneous, TSC-related tumors. Topical treatments were compared with injected rapamycin and topical vehicle. Rapamycin levels in blood and tumors were measured to assess systemic drug levels in all cohorts.
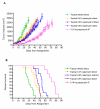
Results: Treatment with topical rapamycin improved survival and reduced tumor growth. Topical rapamycin treatment resulted in systemic drug levels within the known therapeutic range and was not as effective as injected rapamycin.
Conclusion: Topical rapamycin inhibits TSC-related tumor growth. These findings could lead to a novel treatment approach for facial angiofibromas and other TSC skin lesions.
Figures


References
-
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ. Mutational Analysis in a Cohort of 224 Tuberous Sclerosis Patients Indicates Increased Severity of TSC2, Compared with TSC1, Disease in Multiple Organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. - DOI - PMC - PubMed
-
- Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, Sethuraman G, Colby TV, Kwiatkowski DJ, McCormack FX. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–668. - PubMed
-
- Gomez MR, Sampson JR, Whittemore VH. The tuberous sclerosis complex. Third Ed. Oxford, England, Oxford University Press; 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

