Regulation of myoblast motility and fusion by the CXCR4-associated sialomucin, CD164
- PMID: 18227060
- PMCID: PMC2276390
- DOI: 10.1074/jbc.M706730200
Regulation of myoblast motility and fusion by the CXCR4-associated sialomucin, CD164
Abstract
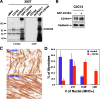
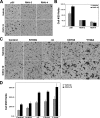
Myoblast fusion is fundamental to the development and regeneration of skeletal muscle. To fuse, myoblasts undergo cell-cell recognition and adhesion and merger of membranes between apposing cells. Cell migration must occur in advance of these events to bring myoblasts into proximity, but the factors that regulate myoblast motility are not fully understood. CD164 is a cell surface sialomucin that is targeted to endosomes and lysosomes via its intracellular region. In hematopoietic progenitor cells, CD164 forms complexes with the motility-stimulating chemokine receptor, CXCR4, in response to the CXCR4 ligand, CXCL12/SDF-1 (Forde, S., Tye, B. J., Newey, S. E., Roubelakis, M., Smythe, J., McGuckin, C. P., Pettengell, R., and Watt, S. M. (2007) Blood 109, 1825-1833). We have previously shown that CD164 stimulates myotube formation in vitro. We report here that CD164 is associated with CXCR4 in C2C12 myoblasts. Cells in which CD164 levels are increased or decreased via overexpression or RNA interference-mediated knockdown, respectively, show enhanced or reduced myotube formation and cell migration, the latter both basally and in response to CXCL12/SDF-1. Furthermore, expression of CD164 cytoplasmic tail mutants that alter the endosome/lysosome targeting sequence and, consequently, the subcellular localization in myoblasts, reveals a similar correlation between cell motility and myotube formation. Finally, Cd164 mRNA is expressed in the dorsal somite (the early myogenic compartment of the mouse embryo) and in premuscle masses. Taken together, these results suggest that CD164 is a regulator of myoblast motility and that this property contributes to its ability to promote myoblast fusion into myotubes.
Figures





References
-
- Tajbakhsh, S., and Buckingham, M. (2000) Curr. Top. Dev. Biol. 48 225–268 - PubMed
-
- Horsley, V., and Pavlath, G. K. (2004) Cells Tissues Organs 176 67–78 - PubMed
-
- Perry, R. L. S., and Rudnicki, M. A. (2000) Front. Biosci. 5 d750–767 - PubMed
-
- Tapscott, S. J. (2005) Development (Camb.) 132 2685–2695 - PubMed
-
- Krauss, R. S., Cole, F., Gaio, U., Takaesu, G., Zhang, W., and Kang, J. S. (2005) J. Cell Sci. 118 2355–2362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

