Regulation of nuclear lamin polymerization by importin alpha
- PMID: 18227062
- PMCID: PMC2417177
- DOI: 10.1074/jbc.M709572200
Regulation of nuclear lamin polymerization by importin alpha
Abstract
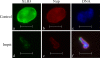
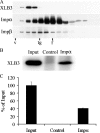
Nuclear lamins are integral components of the nuclear envelope and are important for the regulation of many aspects of nuclear function, including gene transcription and DNA replication. During interphase, the lamins form an intranuclear intermediate filament network that must be disassembled and reassembled when cells divide. Little is known about factors regulating this assembly/disassembly cycle. Using in vitro nuclear assembly and lamin assembly assays, we have identified a role for the nuclear transport factor importin alpha in the regulation of lamin assembly. Exogenous importin alpha inhibited nuclear lamin assembly in Xenopus interphase egg nuclear assembly assays. Fractionation of the egg extract used for nuclear assembly identified a high molecular weight complex containing the major egg lamin, XLB3, importin alpha, and importin beta. This complex could be dissociated by RanGTP or a competing nuclear localization sequence, indicating that lamin assembly is Ran- and importin alpha-dependent in the egg extract. We show that the addition of importin alpha to purified lamin B3 prevents the assembly of lamins in solution. Lamin assembly assays show that importin alpha prevents the self-association of lamins required to assemble lamin filaments into the typical paracrystals formed in vitro. These results suggest a role for importin alpha in regulating lamin assembly and possibly modulating the interactions of lamins with lamin-binding proteins.
Figures
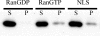
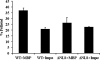

References
-
- Burke, B. (1990) Curr. Opin. Cell Biol. 2 514-520 - PubMed
-
- Mattout, A., Dechat, T., Adam, S. A., Goldman, R. D., and Gruenbaum, Y. (2006) Curr. Opin. Cell Biol. 18 335-341 - PubMed
-
- Dorner, D., Gotzmann, J., and Foisner, R. (2007) FEBS J. 274 1362-1373 - PubMed
-
- Shumaker, D. K., Kuczmarski, E. R., and Goldman, R. D. (2003) Curr. Opin. Cell Biol. 15 358-366 - PubMed
-
- Nakajima, N., and Abe, K. (1995) FEBS Lett. 365 108-114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

