Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption
- PMID: 18227071
- PMCID: PMC2442344
- DOI: 10.1074/jbc.M709712200
Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption
Abstract
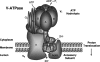
Solubilization of mineralized bone by osteoclasts is largely dependent on the acidification of the extracellular resorption lacuna driven by the vacuolar (H+)-ATPases (V-ATPases) polarized within the ruffled border membranes. V-ATPases consist of two functionally and structurally distinct domains, V(1) and V(0). The peripheral cytoplasmically oriented V(1) domain drives ATP hydrolysis, which necessitates the translocation of protons across the integral membrane bound V(0) domain. Here, we demonstrate that an accessory subunit, Ac45, interacts with the V(0) domain and contributes to the vacuolar type proton pump-mediated function in osteoclasts. Consistent with its role in intracellular acidification, Ac45 was found to be localized to the ruffled border region of polarized resorbing osteoclasts and enriched in pH-dependent endosomal compartments that polarized to the ruffled border region of actively resorbing osteoclasts. Interestingly, truncation of the 26-amino acid residue cytoplasmic tail of Ac45, which encodes an autonomous internalization signal, was found to impair bone resorption in vitro. Furthermore, biochemical analysis revealed that although both wild type Ac45 and mutant were capable of associating with subunits a3, c, c'', and d, deletion of the cytoplasmic tail altered its binding proximity with a3, c'', and d. In all, our data suggest that the cytoplasmic terminus of Ac45 contains elements necessary for its proper interaction with V(0) domain and efficient osteoclastic bone resorption.
Figures




Similar articles
-
V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption.J Bone Miner Res. 2012 Aug;27(8):1695-707. doi: 10.1002/jbmr.1623. J Bone Miner Res. 2012. PMID: 22467241 Free PMC article.
-
Versatile roles of V-ATPases accessory subunit Ac45 in osteoclast formation and function.PLoS One. 2011;6(11):e27155. doi: 10.1371/journal.pone.0027155. Epub 2011 Nov 4. PLoS One. 2011. PMID: 22087256 Free PMC article.
-
Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis.Histol Histopathol. 2007 Apr;22(4):443-54. doi: 10.14670/HH-22.443. Histol Histopathol. 2007. PMID: 17290355 Review.
-
Inhibition of osteoclast bone resorption by disrupting vacuolar H+-ATPase a3-B2 subunit interaction.J Biol Chem. 2010 Nov 26;285(48):37476-90. doi: 10.1074/jbc.M110.123281. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837476 Free PMC article.
-
V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption.Int J Biochem Cell Biol. 2012 Sep;44(9):1422-35. doi: 10.1016/j.biocel.2012.05.014. Epub 2012 May 29. Int J Biochem Cell Biol. 2012. PMID: 22652318 Review.
Cited by
-
ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation.Nat Commun. 2016 May 27;7:11600. doi: 10.1038/ncomms11600. Nat Commun. 2016. PMID: 27231034 Free PMC article.
-
ATP6AP1 promotes cell proliferation and tamoxifen resistance in luminal breast cancer by inducing autophagy.Cell Death Dis. 2025 Mar 25;16(1):201. doi: 10.1038/s41419-025-07534-y. Cell Death Dis. 2025. PMID: 40133274 Free PMC article.
-
Ponicidin Treatment Improved the Cell Proliferation, Differentiation, and Calcium Mineralization on the Osteoblast-Like MG-63 Cells.Appl Biochem Biotechnol. 2022 Sep;194(9):3860-3870. doi: 10.1007/s12010-022-03927-3. Epub 2022 May 12. Appl Biochem Biotechnol. 2022. PMID: 35556208
-
The emerging role of furin in neurodegenerative and neuropsychiatric diseases.Transl Neurodegener. 2022 Aug 23;11(1):39. doi: 10.1186/s40035-022-00313-1. Transl Neurodegener. 2022. PMID: 35996194 Free PMC article. Review.
-
Calmodulin interacts with Rab3D and modulates osteoclastic bone resorption.Sci Rep. 2016 Nov 29;6:37963. doi: 10.1038/srep37963. Sci Rep. 2016. PMID: 27897225 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

