The Caenorhabditis elegans K10C2.4 gene encodes a member of the fumarylacetoacetate hydrolase family: a Caenorhabditis elegans model of type I tyrosinemia
- PMID: 18227072
- PMCID: PMC2431024
- DOI: 10.1074/jbc.M708341200
The Caenorhabditis elegans K10C2.4 gene encodes a member of the fumarylacetoacetate hydrolase family: a Caenorhabditis elegans model of type I tyrosinemia
Abstract
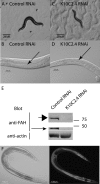
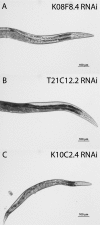
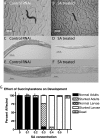
In eukaryotes and many bacteria, tyrosine is degraded to produce energy via a five-step tyrosine degradation pathway. Mutations affecting the tyrosine degradation pathway are also of medical importance as mutations affecting enzymes in the pathway are responsible for type I, type II, and type III tyrosinemia. The most severe of these is type I tyrosinemia, which is caused by mutations affecting the last enzyme in the pathway, fumarylacetoacetate hydrolase (FAH). So far, tyrosine degradation in the nematode Caenorhabditis elegans has not been studied; however, genes predicted to encode enzymes in this pathway have been identified in several microarray, proteomic, and RNA interference (RNAi) screens as perhaps being involved in aging and the control of protein folding. We sought to identify and characterize the genes in the worm tyrosine degradation pathway as an initial step in understanding these findings. Here we describe the characterization of the K10C2.4, which encodes a homolog of FAH. RNAi directed against K10C2.4 produces a lethal phenotype consisting of death in young adulthood, extensive damage to the intestine, impaired fertility, and activation of oxidative stress and endoplasmic stress response pathways. This phenotype is due to alterations in tyrosine metabolism as increases in dietary tyrosine enhance it, and inhibition of upstream enzymes in tyrosine degradation with RNAi or genetic mutations reduces the phenotype. We also use our model to identify genes that suppress the damage produced by K10C2.4 RNAi in a pilot genetic screen. Our results establish worms as a model for the study of type I tyrosinemia.
Figures





Similar articles
-
Structural and functional analysis of missense mutations in fumarylacetoacetate hydrolase, the gene deficient in hereditary tyrosinemia type 1.J Biol Chem. 2001 May 4;276(18):15225-31. doi: 10.1074/jbc.M009341200. Epub 2001 Jan 22. J Biol Chem. 2001. PMID: 11278491
-
Fumarylacetoacetate Hydrolase Knock-out Rabbit Model for Hereditary Tyrosinemia Type 1.J Biol Chem. 2017 Mar 17;292(11):4755-4763. doi: 10.1074/jbc.M116.764787. Epub 2017 Jan 4. J Biol Chem. 2017. PMID: 28053091 Free PMC article.
-
Molecular Aspects of the FAH Mutations Involved in HT1 Disease.Adv Exp Med Biol. 2017;959:25-48. doi: 10.1007/978-3-319-55780-9_3. Adv Exp Med Biol. 2017. PMID: 28755182 Review.
-
Point mutations in the murine fumarylacetoacetate hydrolase gene: Animal models for the human genetic disorder hereditary tyrosinemia type 1.Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):641-5. doi: 10.1073/pnas.98.2.641. Proc Natl Acad Sci U S A. 2001. PMID: 11209059 Free PMC article.
-
The genetic tyrosinemias.Am J Med Genet C Semin Med Genet. 2006 May 15;142C(2):121-6. doi: 10.1002/ajmg.c.30092. Am J Med Genet C Semin Med Genet. 2006. PMID: 16602095 Review.
Cited by
-
Beyond Proteostasis: Lipid Metabolism as a New Player in ER Homeostasis.Metabolites. 2021 Jan 14;11(1):52. doi: 10.3390/metabo11010052. Metabolites. 2021. PMID: 33466824 Free PMC article. Review.
-
Retrofitting ampicillin resistant vectors by recombination for use in generating C. elegans transgenic animals by bombardment.Plasmid. 2009 Sep;62(2):140-5. doi: 10.1016/j.plasmid.2009.06.001. Epub 2009 Jun 9. Plasmid. 2009. PMID: 19520111 Free PMC article.
-
Improved vectors for selection of transgenic Caenorhabditis elegans.Methods Mol Biol. 2013;940:87-102. doi: 10.1007/978-1-62703-110-3_8. Methods Mol Biol. 2013. PMID: 23104336 Free PMC article.
-
LC-MS proteomics analysis of the insulin/IGF-1-deficient Caenorhabditis elegans daf-2(e1370) mutant reveals extensive restructuring of intermediary metabolism.J Proteome Res. 2014 Apr 4;13(4):1938-56. doi: 10.1021/pr401081b. Epub 2014 Mar 3. J Proteome Res. 2014. PMID: 24555535 Free PMC article.
-
TATN-1 mutations reveal a novel role for tyrosine as a metabolic signal that influences developmental decisions and longevity in Caenorhabditis elegans.PLoS Genet. 2013;9(12):e1004020. doi: 10.1371/journal.pgen.1004020. Epub 2013 Dec 19. PLoS Genet. 2013. PMID: 24385923 Free PMC article.
References
-
- Knox, W. E. (1955) Methods in Enzymology, pp. 287-300 Academic Press, New York
-
- Moran, G. R. (2005) Arch. Biochem. Biophys. 433 117-128 - PubMed
-
- Mitchell, G. A., Grompe, M., Lambert, M., and Tanguay, R. M. (2001) in The Metabolic and Molecular Basis of Inherited Disease (Scriver, C. R., ed), pp. 1777-1805 McGraw-Hill, New York
-
- La Du, B. N., Zannoni, V. G., Laster, L., and Seegmiller, J. E. (1958) J. Biol. Chem. 230 251-260 - PubMed
-
- Goldsmith, L. A., Kang, E., Bienfang, D. C., Jimbow, K., Gerald, P., and Baden, H. P. (1973) J. Pediatr. 83 798-805 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

