NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain
- PMID: 18227174
- PMCID: PMC2292861
- DOI: 10.1128/IAI.00745-07
NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain
Abstract
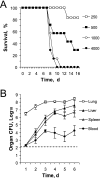
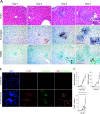
Host innate immune responses to many intracellular pathogens include the formation of inflammatory granulomas that are thought to provide a physical barrier between the microbe and host. Because two common features of infections with the live vaccine strain (LVS) of Francisella tularensis within the mouse liver are the formation of granulomas and the production of gamma interferon (IFN-gamma), we have asked what role IFN-gamma plays in hepatic granuloma formation and function. Francisella antigens were predominantly localized within granulomas of the livers of mice infected with F. tularensis LVS 4 days postinfection. Hepatic granulomas also contained large numbers of dying cells, some of which coexpressed the F4/80 macrophage antigen and activated caspase-3. IFN-gamma-deficient mice did not form normal numbers of hepatic granulomas and showed widely disseminated Francisella antigens within the liver. The incidence of cell death within hepatic granulomas also decreased significantly in the absence of IFN-gamma. Inducible NO synthase (iNOS) expression was restricted to the granulomas of wild-type mice but was not seen for IFN-gamma-deficient mice. Cell death within granulomas was also significantly decreased for iNOS-deficient mice. The predominant IFN-gamma-expressing cells in the liver were NK cells. Depleting NK cells resulted in the expression of bacterial antigens and iNOS outside the granulomas and the appearance of extensive hepatic focal necrosis. These findings indicate that IFN-gamma and hepatic NK cells that are activated during F. tularensis LVS infections regulate hepatic granuloma formation, the spatial containment of infection, the expression of iNOS, and the induction of cell death within the liver.
Figures




References
-
- Anthony, L. S., E. Ghadirian, F. P. Nestel, and P. A. Kongshavn. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7421-428. - PubMed
-
- Chen, W., R. KuoLee, H. Shen, and J. W. Conlan. 2004. Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb. Pathog. 36311-318. - PubMed
-
- Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 1766888-6899. - PubMed
-
- Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34239-248. - PubMed
-
- Conlan, J. W., R. KuoLee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32127-134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

