Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro
- PMID: 18227187
- PMCID: PMC2292515
- DOI: 10.1128/AAC.01228-07
Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro
Abstract
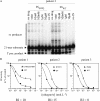
Raltegravir (MK-0518) is a potent inhibitor of human immunodeficiency virus (HIV) integrase and is clinically effective against viruses resistant to other classes of antiretroviral agents. However, it can select mutations in the HIV integrase gene. Nine heavily pretreated patients who received salvage therapy including raltegravir and who subsequently developed virological failure under raltegravir therapy were studied. For each patient, the sequences of the integrase-coding region were determined and compared to that at the beginning of the treatment. Four different mutation profiles were identified in these nine patients: E92Q, G140S Q148H, N155H, and E157Q mutations. For four patients, each harboring a different profile, the wild-type and mutated integrases were produced, purified, and assayed in vitro. All the mutations identified altered the activities of integrase protein: both 3' processing and strand transfer activities were moderately affected in the E92Q mutant; strand transfer was markedly impaired in the N155H mutant; both activities were strongly impaired in the G140S Q148H mutant; and the E157Q mutant was almost completely inactive. The sensitivities of wild-type and mutant integrases to raltegravir were compared. The E92Q and G140S Q148H profiles were each associated with a 7- to 8-fold decrease in sensitivity, and the N155H mutant was more than 14-fold less sensitive to raltegravir. At least four genetic profiles (E92Q, G140S Q148H, N155H, and E157Q) can be associated with in vivo treatment failure and resistance to raltegravir. These mutations led to strong impairment of enzymes in vitro in the absence of raltegravir: strand transfer activity was affected, and in some cases 3' processing was also impaired.
Figures

References
-
- Ceccherini-Silberstein, F., I. Malet, L. Fabeni, V. Svicher, C. Gori, S. Dimonte, S. Bono, A. Artese, R. D'Arrigo, C. Katlama, A. Antinori, A. d'Arminio Monforte, V. Calvez, A. G. Marcelin, and C. F. Perno on behalf of the EuroGene HIV Network. 2007. Abstr. 5th Eur. HIV Drug Resistance Workshop, abstr. 52.
-
- Cherepanov, P., G. Maertens, P. Proost, B. Devreese, J. Van Beeumen, Y. Engelborghs, E. De Clercq, and Z. Debyser. 2003. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 278:372-381. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

