Pulmonary arterial remodeling induced by a Th2 immune response
- PMID: 18227220
- PMCID: PMC2271018
- DOI: 10.1084/jem.20071008
Pulmonary arterial remodeling induced by a Th2 immune response
Abstract
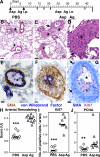
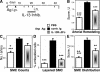
Pulmonary arterial remodeling characterized by increased vascular smooth muscle density is a common lesion seen in pulmonary arterial hypertension (PAH), a deadly condition. Clinical correlation studies have suggested an immune pathogenesis of pulmonary arterial remodeling, but experimental proof has been lacking. We show that immunization and prolonged intermittent challenge via the airways with either of two different soluble antigens induced severe muscularization in small- to medium-sized pulmonary arteries. Depletion of CD4(+) T cells, antigen-specific T helper type 2 (Th2) response, or the pathogenic Th2 cytokine interleukin 13 significantly ameliorated pulmonary arterial muscularization. The severity of pulmonary arterial muscularization was associated with increased numbers of epithelial cells and macrophages that expressed a smooth muscle cell mitogen, resistin-like molecule alpha, but surprisingly, there was no correlation with pulmonary hypertension. Our data are the first to provide experimental proof that the adaptive immune response to a soluble antigen is sufficient to cause severe pulmonary arterial muscularization, and support the clinical observations in pediatric patients and in companion animals that muscularization represents one of several injurious events to the pulmonary artery that may collectively contribute to PAH.
Figures



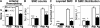
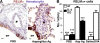

References
-
- Heath, D., and J.E. Edwards. 1958. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 18:533–547. - PubMed
-
- Wagenvoort, C.A. 1970. The pathology of primary pulmonary hypertension. J. Pathol. 101:Pi. - PubMed
-
- Reid, L.M. 1979. The pulmonary circulation: remodeling in growth and disease. The 1978 J. Burns Amberson lecture. Am. Rev. Respir. Dis. 119:531–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

