Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials
- PMID: 18227449
- PMCID: PMC2211353
- DOI: 10.1503/cmaj.070693
Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials
Abstract
Background: Concern has been raised about the efficacy of antidepressant therapy for major depression in adults. We undertook a systematic review of published and unpublished clinical trial data to determine the effectiveness and acceptability of paroxetine.
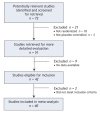
Methods: We searched the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register, the Cochrane Central Register of Controlled Trials, the GlaxoSmithKline Clinical Trial Register, MEDLINE and EMBASE up to December 2006. Published and unpublished randomized trials comparing paroxetine with placebo in adults with major depression were eligible for inclusion. We selected the proportion of patients who left a study early for any reason as the primary outcome measure because it represents a hard measure of treatment effectiveness and acceptability.
Results: We included in our review 29 published and 11 unpublished clinical trials, with a total of 3704 patients who received paroxetine and 2687 who received with placebo. There was no difference between paroxetine and placebo in terms of the proportion of patients who left the study early for any reason (random effect relative risk [RR] 0.99, 99% confidence interval [CI] 0.88-1.11). Paroxetine was more effective than placebo, with fewer patients who did not experience improvement in symptoms of at least 50% (random effect RR 0.83, 99% CI 0.77-0.90). Significantly more patients in the paroxetine group than in the placebo group left their respective studies because of side effects (random effect RR 1.77, 95% CI 1.44-2.18) or experienced suicidal tendencies (odds ratio 2.55, 95% CI 1.17-5.54).
Interpretation: Among adults with moderate to severe major depression in the clinical trials we reviewed, paroxetine was not superior to placebo in terms of overall treatment effectiveness and acceptability. These results were not biased by selective inclusion of published studies.
Figures




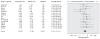
Comment in
-
Effectiveness of selective serotonin reuptake inhibitors.CMAJ. 2008 Apr 22;178(9):1185; author reply 1185-6. doi: 10.1503/cmaj.1080023. CMAJ. 2008. PMID: 18427097 Free PMC article. No abstract available.
-
Review: paroxetine and placebo do not differ for treatment discontinuation in major depression.Evid Based Med. 2008 Aug;13(4):110. doi: 10.1136/ebm.13.4.110. Evid Based Med. 2008. PMID: 18667669 No abstract available.
References
-
- National Collaborating Centre for Mental Health. Depression (amended). Management of depression in primary and secondary care. London (UK): National Institute for Health and Clinical Excellence; 2007.
-
- Bauer M, Whybrow PC, Angst J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: Maintenance treatment of major depressive disorder and treatment of chronic depressive disorders and subthreshold depressions. World J Biol Psychiatry 2002;3:69-86. - PubMed
-
- Moncrieff J. The antidepressant debate. Br J Psychiatry 2002;180:193-4. - PubMed
-
- Keller MB, Lavori PW. The adequacy of treating depression. J Nerv Ment Dis 1988;176:471-4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
