Molecular insights into human daily behavior
- PMID: 18227513
- PMCID: PMC2234191
- DOI: 10.1073/pnas.0707772105
Molecular insights into human daily behavior
Abstract
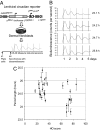
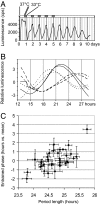
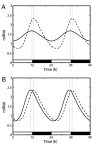
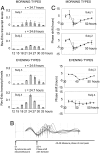
Human beings exhibit wide variation in their timing of daily behavior. We and others have suggested previously that such differences might arise because of alterations in the period length of the endogenous human circadian oscillator. Using dermal fibroblast cells from skin biopsies of 28 subjects of early and late chronotype (11 "larks" and 17 "owls"), we have studied the circadian period lengths of these two groups, as well as their ability to phase-shift and entrain to environmental and chemical signals. We find not only period length differences between the two classes, but also significant changes in the amplitude and phase-shifting properties of the circadian oscillator among individuals with identical "normal" period lengths. Mathematical modeling shows that these alterations could also account for the extreme behavioral phenotypes of these subjects. We conclude that human chronotype may be influenced not only by the period length of the circadian oscillator, but also by cellular components that affect its amplitude and phase. In many instances, these changes can be studied at the molecular level in primary dermal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma. 2004;113:103–112. - PubMed
-
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. - PubMed
-
- Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: A nationwide study of 8753 adult twin pairs. J Sleep Res. 2007;16:156–162. - PubMed
-
- Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. - PubMed
-
- Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

