Incorporating the effects of transcytolemmal water exchange in a reference region model for DCE-MRI analysis: theory, simulations, and experimental results
- PMID: 18228592
- PMCID: PMC2692327
- DOI: 10.1002/mrm.21449
Incorporating the effects of transcytolemmal water exchange in a reference region model for DCE-MRI analysis: theory, simulations, and experimental results
Abstract
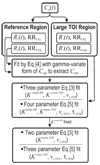
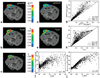
Models have been developed for the analysis of dynamic contrast-enhanced MRI (DCE-MRI) data that do not require direct measurements of the arterial input function; such methods are referred to as reference region models. These models typically return estimates of the volume transfer constant (K(trans)) and the extravascular extracellular volume fraction (v(e)). To date such models have assumed a linear relationship between the measured R(1) ( identical with 1/T(1)) and the concentration of contrast agent, a transformation referred to as the fast exchange limit, but this assumption is not valid for all concentrations of an agent. A theory for DCE-MRI reference region models which accounts for water exchange is presented, evaluated in simulations, and applied in tumor-bearing mice. Using reasonable parameter values, simulations show that the assumption of fast exchange can underestimate K(trans) and v(e) by up to 82% and 46%, respectively. By analyzing a large region of interest and a single voxel the new model can return parameters within approximately +/-10% and +/-25%, respectively, of their true values. Analysis of experimental data shows that the new approach returns K(trans) and v(e) values that are up to 90% and 73%, respectively, greater than conventional fast exchange analyses.
(c) 2008 Wiley-Liss, Inc.
Figures





References
-
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HBW, Lee T-Y, Mayr NA, Parker GJM, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. - PubMed
-
- Li KL, Wilmes LJ, Henry RG, Pallavicini MG, Park JW, Hu-Lowe DD, McShane TM, Shalinsky DR, Fu YJ, Brasch RC, Hylton NM. Heterogeneity in the angiogenic response of a BT474 human breast cancer to a novel vascular endothelial growth factor-receptor tyrosine kinase inhibitor: assessment by voxel analysis of dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2005;22:511–519. - PubMed
-
- Wang B, Gao ZQ, Yan X. Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol. 2005;46:353–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

