Oxidative stress and cellular stress response in diabetic nephropathy
- PMID: 18229449
- PMCID: PMC2134792
- DOI: 10.1379/csc-270.1
Oxidative stress and cellular stress response in diabetic nephropathy
Abstract
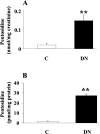
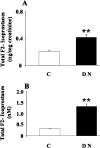
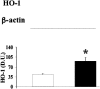
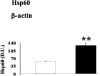
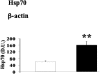
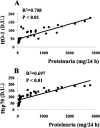
Oxidative stress has been suggested to play a main role in the pathogenesis of type 2 diabetes mellitus and its complications. As a consequence of this increased oxidative status, a cellular-adaptive response occurs requiring functional chaperones, antioxidant production, and protein degradation. This study was designed to evaluate systemic oxidative stress and cellular stress response in patients suffering from type 2 diabetes-induced nephropathy and in age-matched healthy subjects. Systemic oxidative stress has been evaluated by measuring advanced glycation end-products (pentosidine), protein oxidation (protein carbonyls [DNPH]), and lipid oxidation (4-hydroxy-2-nonenal [HNE] and F2-isoprostanes) in plasma, lymphocytes, and urine, whereas the lymphocyte levels of the heat shock proteins (Hsps) heme oxygenase-1 (HO-1), Hsp70, and Hsp60 as well as thioredoxin reductase-1 (TrxR-1) have been measured to evaluate the systemic cellular stress response. We found increased levels of pentosidine (P < 0.01), DNPH (P < 0.05 and P < 0.01), HNE (P < 0.05 and P < 0.01), and F2-isoprostanes (P < 0.01) in all the samples from type 2 diabetic patients with nephropathy with respect to control group. This was paralleled by a significant induction of cellular HO-1, Hsp60, Hsp70, and TrxR-1 (P < 0.05 and P < 0.01). A significant upregulation of both HO-1 and Hsp70 has been detected also in lymphocytes from type 2 diabetic patients without uraemia. Significant positive correlations between DNPH and Hsp60, as well as between the degree of renal failure and HO-1 or Hsp70, also have been found in diabetic uremic subjects. In conclusion, patients affected by type 2 diabetes complicated with nephropathy are under condition of systemic oxidative stress, and the induction of Hsp and TrxR-1 is a maintained response in counteracting the intracellular pro-oxidant status.
Figures
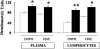



References
-
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345.0012-1797(2003)052[2338:IHSPAH]2.0.CO;2 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
