Amantadine-induced conformational and dynamical changes of the influenza M2 transmembrane proton channel
- PMID: 18230730
- PMCID: PMC2234170
- DOI: 10.1073/pnas.0711500105
Amantadine-induced conformational and dynamical changes of the influenza M2 transmembrane proton channel
Abstract
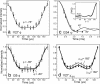
The M2 protein of influenza A virus forms a transmembrane proton channel important for viral infection and replication. Amantadine blocks this channel, thus inhibiting viral replication. Elucidating the high-resolution structure of the M2 protein and its change upon amantadine binding is crucial for designing antiviral drugs to combat the growing resistance of influenza A viruses against amantadine. We used magic-angle-spinning solid-state NMR to determine the conformation and dynamics of the transmembrane domain of the protein M2TMP in the apo- and amantadine-bound states in lipid bilayers. (13)C chemical shifts and torsion angles of the protein in 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine (DLPC) bilayers indicate that M2TMP is alpha-helical in both states, but the average conformation differs subtly, especially at the G34-I35 linkage and V27 side chain. In the liquid-crystalline membrane, the complexed M2TMP shows dramatically narrower lines than the apo peptide. Analysis of the homogeneous and inhomogeneous line widths indicates that the apo-M2TMP undergoes significant microsecond-time scale motion, and amantadine binding alters the motional rates, causing line-narrowing. Amantadine also reduces the conformational heterogeneity of specific residues, including the G34/I35 pair and several side chains. Finally, amantadine causes the helical segment N-terminal to G34 to increase its tilt angle by 3 degrees , and the G34-I35 torsion angles cause a kink of 5 degrees in the amantadine-bound helix. These data indicate that amantadine affects the M2 proton channel mainly by changing the distribution and exchange rates among multiple low-energy conformations and only subtly alters the average conformation and orientation. Amantadine-resistant mutations thus may arise from binding-incompetent changes in the conformational equilibrium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
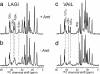
 13C DQ-filtered spectra of M2TMP in DLPC bilayers without (black) and with (red) amantadine at 243 K. Intraresidue connectivities and cross-peaks with chemical-shift changes are indicated. (a) LAGI. (b) VAIL. (c) Selected 1D cross-sections that exhibit line-narrowing and chemical-shift changes upon amantadine binding. The G34α trace was extracted from 1D CP spectra.
13C DQ-filtered spectra of M2TMP in DLPC bilayers without (black) and with (red) amantadine at 243 K. Intraresidue connectivities and cross-peaks with chemical-shift changes are indicated. (a) LAGI. (b) VAIL. (c) Selected 1D cross-sections that exhibit line-narrowing and chemical-shift changes upon amantadine binding. The G34α trace was extracted from 1D CP spectra.

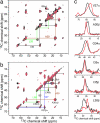
 1H dipolar coupling of unoriented M2TMP in DLPC bilayers with amantadine (filled squares, thick line). For comparison, the apo peptide data published recently are superimposed (open squares, thin line) (31). (a) V28. (b) A30. (c) PISA wheels of M2TMP constructed from the δ// N
1H dipolar coupling of unoriented M2TMP in DLPC bilayers with amantadine (filled squares, thick line). For comparison, the apo peptide data published recently are superimposed (open squares, thin line) (31). (a) V28. (b) A30. (c) PISA wheels of M2TMP constructed from the δ// N H dipolar couplings and δ⊥ 15N anisotropic shifts. The data fit to a wheel with a tilt angle τ of 38° (thick line). The apo peptide shows a τ = 35° (open symbols, thin line) (31).
H dipolar couplings and δ⊥ 15N anisotropic shifts. The data fit to a wheel with a tilt angle τ of 38° (thick line). The apo peptide shows a τ = 35° (open symbols, thin line) (31).
References
-
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. - PubMed
-
- Pinto LH, Lamb RA. Controlling influenza virus replication by inhibiting its proton flow. Mol Bio Syst. 2007;3:18–23. - PubMed
-
- Lamb RA, Holsinger KJ, Pinto LH. In: Cellular Receptors of Animal Viruses. Wemmer E, editor. Plainview, NY: Cold Spring Harbor Lab Press; 1994. pp. 303–321.
-
- Bright RA, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet. 2005;366:1175–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

