Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy
- PMID: 18230737
- PMCID: PMC2234157
- DOI: 10.1073/pnas.0707654105
Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy
Abstract
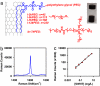
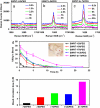
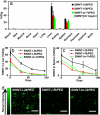
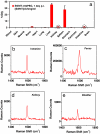
Carbon nanotubes are promising new materials for molecular delivery in biological systems. The long-term fate of nanotubes intravenously injected into animals in vivo is currently unknown, an issue critical to potential clinical applications of these materials. Here, using the intrinsic Raman spectroscopic signatures of single-walled carbon nanotubes (SWNTs), we measured the blood circulation of intravenously injected SWNTs and detect SWNTs in various organs and tissues of mice ex vivo over a period of three months. Functionalization of SWNTs by branched polyethylene-glycol (PEG) chains was developed, enabling thus far the longest SWNT blood circulation up to 1 day, relatively low uptake in the reticuloendothelial system (RES), and near-complete clearance from the main organs in approximately 2 months. Raman spectroscopy detected SWNT in the intestine, feces, kidney, and bladder of mice, suggesting excretion and clearance of SWNTs from mice via the biliary and renal pathways. No toxic side effect of SWNTs to mice was observed in necropsy, histology, and blood chemistry measurements. These findings pave the way to future biomedical applications of carbon nanotubes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Whitesides GM. The “right” size in nanobiotechnology. Nat Biotech. 2003;21:1161–1165. - PubMed
-
- Service RF. Nanotechnology takes aim at cancer. Science. 2005;310:1132–1134. - PubMed
-
- Sinha R, Kim GJ, Nie SM, Shin DM. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–1917. - PubMed
-
- Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotech. 2004;22:969–976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

