Hsc70 focus formation at the periphery of HSV-1 transcription sites requires ICP27
- PMID: 18231578
- PMCID: PMC2200795
- DOI: 10.1371/journal.pone.0001491
Hsc70 focus formation at the periphery of HSV-1 transcription sites requires ICP27
Abstract
Background: The cellular chaperone protein Hsc70, along with components of the 26S proteasome and ubiquitin-conjugated proteins have been shown to be sequestered in discrete foci in the nuclei of herpes simplex virus 1 (HSV-1) infected cells. We recently reported that cellular RNA polymerase II (RNAP II) undergoes proteasomal degradation during robust HSV-1 transcription, and that the immediate early protein ICP27 interacts with the C-terminal domain and is involved in the recruitment of RNAP II to viral transcription/replication compartments.
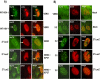
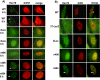
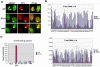
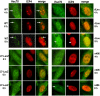
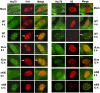
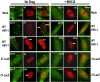
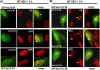
Methodology/principle findings: Here we show that ICP27 also interacts with Hsc70, and is required for the formation of Hsc70 nuclear foci. During infection with ICP27 mutants that are unable to recruit RNAP II to viral replication sites, viral transcript levels were greatly reduced, viral replication compartments were poorly formed and Hsc70 focus formation was curtailed. Further, a dominant negative Hsc70 mutant that cannot hydrolyze ATP, interfered with RNAP II degradation during HSV-1 infection, and an increase in ubiquitinated forms of RNAP II was observed. There was also a decrease in virus yields, indicating that proteasomal degradation of stalled RNAP II complexes during robust HSV-1 transcription and replication benefits viral gene expression.
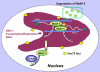
Conclusions/significance: We propose that one function of the Hsc70 nuclear foci may be to serve to facilitate the process of clearing stalled RNAP II complexes from viral genomes during times of highly active transcription.
Conflict of interest statement
Figures



References
-
- Kauppinen KP, Duan F, Wels JI, Manor D. Regulation of the Dbl proto-oncogene by heat shock cognate protein 70 (Hsc70). J Biol Chem. 2005;280:21638–21644. - PubMed
-
- Ungewickell E, Ungewickell H, Holstein SE. Functional interaction of the auxillin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem. 1997;272:19594–19600. - PubMed
-
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nature Cell Biol. 2001;3:100–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

