Anti-human tissue factor antibody ameliorated intestinal ischemia reperfusion-induced acute lung injury in human tissue factor knock-in mice
- PMID: 18231608
- PMCID: PMC2211395
- DOI: 10.1371/journal.pone.0001527
Anti-human tissue factor antibody ameliorated intestinal ischemia reperfusion-induced acute lung injury in human tissue factor knock-in mice
Abstract
Background: Interaction between the coagulation and inflammation systems plays an important role in the development of acute respiratory distress syndrome (ARDS). Anti-coagulation is an attractive option for ARDS treatment, and this has promoted development of new antibodies. However, preclinical trials for these antibodies are often limited by the high cost and availability of non-human primates. In the present study, we developed a novel alternative method to test the role of a humanized anti-tissue factor mAb in acute lung injury with transgenic mice.
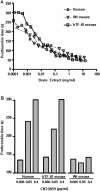
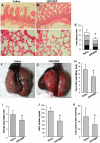
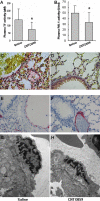
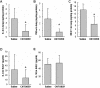
Methodology/principal findings: Human tissue factor knock-in (hTF-KI) transgenic mice and a novel humanized anti-human tissue factor mAb (anti-hTF mAb, CNTO859) were developed. The hTF-KI mice showed a normal and functional expression of hTF. The anti-hTF mAb specifically blocked the pro-coagulation activity of brain extracts from the hTF-KI mice and human, but not from wild type mice. An extrapulmonary ARDS model was used by intestinal ischemia-reperfusion. Significant lung tissue damage in hTF-KI mice was observed after 2 h reperfusion. Administration of CNTO859 (5 mg/kg, i.v.) attenuated the severity of lung tissue injury, decreased the total cell counts and protein concentration in bronchoalveolar lavage fluid, and reduced Evans blue leakage. In addition, the treatment significantly reduced alveolar fibrin deposition, and decreased tissue factor and plasminogen activator inhibitor-1 activity in the serum. This treatment also down-regulated cytokine expression and reduced cell death in the lung.
Conclusions: This novel anti-hTF antibody showed beneficial effects on intestinal ischemia-reperfusion induced acute lung injury, which merits further investigation for clinical usage. In addition, the use of knock-in transgenic mice to test the efficacy of antibodies against human-specific proteins is a novel strategy for preclinical studies.
Conflict of interest statement
Figures
References
-
- Liu M. Searching for acute respiratory distress syndrome genes: aren't we there yet? Am J Respir Crit Care Med. 2005;171:298–299. - PubMed
-
- Rijneveld AW, Weijer S, Bresser P, Florquin S, Vlasuk GP, et al. Local activation of the tissue factor-factor VIIa pathway in patients with pneumonia and the effect of inhibition of this pathway in murine pneumococcal pneumonia. Crit Care Med. 2006;34:1725–1730. - PubMed
-
- Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22:401–404. - PubMed
-
- Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, et al. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med. 2003;167:1200–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

