Cooperative function of Tbx1 and Brn4 in the periotic mesenchyme is necessary for cochlea formation
- PMID: 18231833
- PMCID: PMC2536808
- DOI: 10.1007/s10162-008-0110-6
Cooperative function of Tbx1 and Brn4 in the periotic mesenchyme is necessary for cochlea formation
Abstract
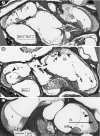
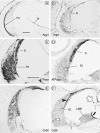
The T-box transcription factor TBX1 has been identified as the major gene responsible for the etiology of velocardiofacial syndrome/DiGeorge syndrome (VCFS/DGS). Conductive hearing loss occurs in a majority of patients with this syndrome, while sensorineural deafness has also been reported in some cases. Mutations in POU3F4/BRN4, a POU domain transcription factor, cause DFN3, an X-linked nonsyndromic form of deafness characterized by mixed conductive and sensorineural hearing loss. Inactivation of the murine orthologues of these genes causes similar defects to those seen in humans and has provided excellent models for the study of inner ear development. Tbx1 and Brn4 are expressed in the mesenchymal cells surrounding the otic vesicle and have been shown to play roles in cochlear outgrowth. Furthermore, expression of Brn4 is reduced in Tbx1 null mutants, suggesting a possible genetic interaction between these genes. To test whether Tbx1 and Brn4 function in a common pathway, mice mutant for both genes were generated and analyzed for inner ear defects. Brn4-;Tbx1+/- mutants displayed a significant reduction in the number of turns of the cochlea compared to Brn4- or Tbx1+/- mice. In addition, Brn4-;Tbx1+/- mice displayed structural defects in the apical cochlea indicative of Mondini dysplasia found in patients with either VCFS/DGS or DFN3. These data establish a genetic interaction between Tbx1 and Brn4 relevant to human disease and indicate a function of these genes in signaling from the periotic mesenchyme to the otic vesicle to direct proper coiling of the cochlear duct.
Figures



References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10979118', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10979118/'}]}
- Arellano B, Ramirez Camacho R, Garcia Berrocal JR, Villamar M, del Castillo I, Moreno F. Sensorineural hearing loss and Mondini dysplasia caused by a deletion at locus DFN3. Arch. Otolaryngol. Head Neck Surg. 126:1065–1069, 2000. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1093/hmg/ddl084', 'is_inner': False, 'url': 'https://doi.org/10.1093/hmg/ddl084'}, {'type': 'PMC', 'value': 'PMC2563157', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2563157/'}, {'type': 'PubMed', 'value': '16600992', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16600992/'}]}
- Arnold JS, Braunstein EM, Ohyama T, Groves AK, Adams JC, Brown MC, Morrow BE. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum. Mol. Genet. 15:1629–1639, 2006a. - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1242/dev.02264', 'is_inner': False, 'url': 'https://doi.org/10.1242/dev.02264'}, {'type': 'PubMed', 'value': '16452092', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16452092/'}]}
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development 133:977–987, 2006b. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1242/dev.000760', 'is_inner': False, 'url': 'https://doi.org/10.1242/dev.000760'}, {'type': 'PubMed', 'value': '17395647', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17395647/'}]}
- Bok J, Dolson DK, Hill P, Ruther U, Epstein DJ, Wu DK. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134:1713–1722, 2007. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1006/dbio.2002.0822', 'is_inner': False, 'url': 'https://doi.org/10.1006/dbio.2002.0822'}, {'type': 'PubMed', 'value': '12435365', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12435365/'}]}
- Chang W, ten Dijke P, Wu DK. BMP pathways are involved in otic capsule formation and epithelial–mesenchymal signaling in the developing chicken inner ear. Dev. Biol. 251:380–394, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

