Development and regeneration of the neonatal digit tip in mice
- PMID: 18234177
- PMCID: PMC2329911
- DOI: 10.1016/j.ydbio.2007.12.025
Development and regeneration of the neonatal digit tip in mice
Abstract
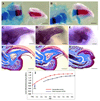
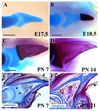
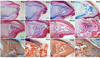
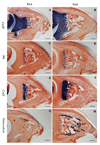
The digit tips of children and rodents are known to regenerate following amputation. The skeletal structure that regenerates is the distal region of the terminal phalangeal bone that is associated with the nail organ. The terminal phalanx forms late in gestation by endochondral ossification and continues to elongate until sexual maturity (8 weeks of age). Postnatal elongation at its distal end occurs by appositional ossification, i.e. direct ossification on the surface of the terminal phalanx, whereas proximal elongation results from an endochondral growth plate. Amputation through the middle of the terminal phalanx regenerates whereas regenerative failure is observed following amputation to remove the distal 2/3 of the bone. Regeneration is characterized by the formation of a blastema of proliferating cells that appear undifferentiated and express Bmp4. Using chondrogenic and osteogenic markers we show that redifferentiation does not occur by endochondral ossification but by the direct ossification of blastema cells that form the rudiment of the digit tip. Once formed the rudiment elongates by appositional ossification in parallel with unamputated control digits. Regenerated digits are consistently shorter than unamputated control digits. Finally, we present a case study of a child who suffered an amputation injury at a proximal level of the terminal phalanx, but failed to regenerate despite conservative treatment and the presence of the nail organ. These clinical and experimental findings expand on previously published observations and initiate a molecular assessment of a mammalian regeneration model.
Figures



References
-
- Allan CH, Fleckman P, Fernandes RJ, Hager B, James J, Wisecarver Z, Satterstrom FK, Gutierrez A, Norman A, Pirrone A, Underwood RA, Rubin BP, Zhang M, Ramay HR, Clark JM. Tissue response and Msx1 expression after human fetal digit tip amputation in vitro. Wound Repair Regen. 2006;14:398–404. - PubMed
-
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. - PubMed
-
- Borgens RB. Mice regrow the tips of their foretoes. Science. 1982;217:747–750. - PubMed
-
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–574. - PubMed
-
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

