Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1)
- PMID: 18234269
- PMCID: PMC2680697
- DOI: 10.1016/j.virol.2007.12.029
Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1)
Abstract
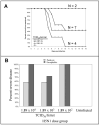
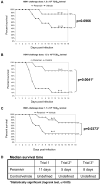
The post-exposure therapeutic efficacy of injectable peramivir against highly pathogenic avian influenza type A H5N1 was evaluated in mice and in ferrets. Seventy to eighty percent of the H5N1-infected peramivir-treated mice, and 70% in the oseltamivir treated mice survived the 15-day study period, as compared to 36% in control (vehicle) group. Ferrets were infected intranasally with H5N1 followed by treatment with multiple doses of peramivir. In two of three trials, a statistically significant increase in survival over a 16-18 day period resulted from peramivir treatment, with improved survival of 40-64% in comparison to mock-treated or untreated animals. Injected peramivir mitigates virus-induced disease, reduces infectious virus titers in the lungs and brains and promotes survival in ferrets infected intranasally with this highly neurovirulent isolate. A single intramuscular peramivir injection protected mice against severe disease outcomes following infection with highly pathogenic avian influenza and multi-dose treatment was efficacious in ferrets.
Figures


Similar articles
-
Efficacy of single intravenous injection of peramivir against influenza B virus infection in ferrets and cynomolgus macaques.Antimicrob Agents Chemother. 2011 Nov;55(11):4961-70. doi: 10.1128/AAC.00412-11. Epub 2011 Aug 15. Antimicrob Agents Chemother. 2011. PMID: 21844317 Free PMC article.
-
Therapeutic efficacy of peramivir against H5N1 highly pathogenic avian influenza viruses harboring the neuraminidase H275Y mutation.Antiviral Res. 2017 Mar;139:41-48. doi: 10.1016/j.antiviral.2016.12.011. Epub 2016 Dec 22. Antiviral Res. 2017. PMID: 28012921
-
Therapeutic activity of intramuscular peramivir in mice infected with a recombinant influenza A/WSN/33 (H1N1) virus containing the H275Y neuraminidase mutation.Antimicrob Agents Chemother. 2012 Aug;56(8):4375-80. doi: 10.1128/AAC.00753-12. Epub 2012 Jun 4. Antimicrob Agents Chemother. 2012. PMID: 22664977 Free PMC article.
-
Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza.Expert Opin Investig Drugs. 2002 Jun;11(6):859-69. doi: 10.1517/13543784.11.6.859. Expert Opin Investig Drugs. 2002. PMID: 12036429 Review.
-
Peramivir and its use in H1N1 influenza.Drugs Today (Barc). 2010 Jun;46(6):399-408. doi: 10.1358/dot.2010.46.6.1459659. Drugs Today (Barc). 2010. PMID: 20571608 Review.
Cited by
-
Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice.Antiviral Res. 2008 Nov;80(2):150-7. doi: 10.1016/j.antiviral.2008.05.012. Epub 2008 Jun 23. Antiviral Res. 2008. PMID: 18573280 Free PMC article.
-
Molecular simulation study of the binding mechanism of [α-PTi2W10O40]7- for its promising broad-spectrum inhibitory activity to FluV-A neuraminidase.Chin Sci Bull. 2010;55(23):2497-2504. doi: 10.1007/s11434-010-3271-8. Epub 2010 Aug 14. Chin Sci Bull. 2010. PMID: 32214733 Free PMC article.
-
A Review of the Antiviral Susceptibility of Human and Avian Influenza Viruses over the Last Decade.Scientifica (Cairo). 2014;2014:430629. doi: 10.1155/2014/430629. Epub 2014 Apr 2. Scientifica (Cairo). 2014. PMID: 24800107 Free PMC article. Review.
-
Novel linear DNA vaccines induce protective immune responses against lethal infection with influenza virus type A/H5N1.Hum Vaccin. 2008 Nov-Dec;4(6):410-9. doi: 10.4161/hv.4.6.6177. Epub 2008 Nov 21. Hum Vaccin. 2008. PMID: 18443425 Free PMC article.
-
Efficacy of single intravenous injection of peramivir against influenza B virus infection in ferrets and cynomolgus macaques.Antimicrob Agents Chemother. 2011 Nov;55(11):4961-70. doi: 10.1128/AAC.00412-11. Epub 2011 Aug 15. Antimicrob Agents Chemother. 2011. PMID: 21844317 Free PMC article.
References
-
- Abed Y, Bourgault AM, Fenton RJ, Morley PJ, Gower D, Owens IJ, Tisdale M, Boivin G. Characterization of 2 influenza A(H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J Infect Dis. 2002;186(8):1074–80. - PubMed
-
- Babu YS, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin TH, Hutchison TL, Elliott AJ, Parker CD, Ananth SL, Horn LL, Laver GW, Montgomery JA. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43(19):3482–6. - PubMed
-
- Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 2006;69(1):39–45. - PubMed
-
- Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45(4):1162–7. - PMC - PubMed
-
- Barnard DL. RWJ-270201 BioCryst Pharmaceuticals/Johnson & Johnson. Curr Opin Investig Drugs. 2000;1(4):421–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

