The detection limit of a Gd3+-based T1 agent is substantially reduced when targeted to a protein microdomain
- PMID: 18234462
- PMCID: PMC2483407
- DOI: 10.1016/j.mri.2007.11.002
The detection limit of a Gd3+-based T1 agent is substantially reduced when targeted to a protein microdomain
Abstract
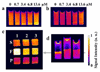
Simple low molecular weight (MW) chelates of Gd(3+) such as those currently used in clinical MRI are considered too insensitive for most molecular imaging applications. Here, we evaluated the detection limit (DL) of a molecularly targeted low MW Gd(3+)-based T(1) agent in a model where the receptor concentration was precisely known. The data demonstrate that receptors clustered together to form a microdomain of high local concentration can be imaged successfully even when the bulk concentration of the receptor is quite low. A GdDO3A-peptide identified by phage display to target the anti-FLAG antibody was synthesized, purified and characterized. T(1-)weighted MR images were compared with the agent bound to antibody in bulk solution and with the agent bound to the antibody localized on agarose beads. Fluorescence competition binding assays show that the agent has a high binding affinity (K(D)=150 nM) for the antibody, while the fully bound relaxivity of the GdDO3A-peptide/anti-FLAG antibody in solution was a relatively modest 17 mM(-1) s(-1). The agent/antibody complex was MR silent at concentrations below approximately 9 microM but was detectable down to 4 microM bulk concentrations when presented to antibody clustered together on the surface of agarose beads. These results provided an estimate of the DLs for other T(1)-based agents with higher fully bound relaxivities or multimeric structures bound to clustered receptor molecules. The results demonstrate that the sensitivity of molecularly targeted contrast agents depends on the local microdomain concentration of the target protein and the molecular relaxivity of the bound complex. A model is presented, which predicts that for a molecularly targeted agent consisting of a single Gd(3+) complex with bound relaxivity of 100 mM(-1) s(-1) or, more reasonably, four tethered Gd(3+) complexes each having a bound relaxivity of 25 mM(-1) s(-1), the DL of a protein microdomain is approximately 690 nM at 9.4 T. These experimental and extrapolated DLs are both well below current literature estimates and suggests that detection of low MW molecularly targeted T(1) agents is not an unrealistic goal.
Figures






References
-
- Tóth É, Helm L, Merbach AE. The chemistry of contrast agents in medical magnetic resonance imaging. In: Merbach AE, Tóth É, editors. Relaxivity of gadolinium(III) complexes: theory and mechanism. Chichester: John Wiley & Sons; 2001. pp. 45–119.
-
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. - PubMed
-
- Aime S, Botta M, Fasano M, Terreno E. Prototropic and water-exchange processes in aqueous solutions of Gd(III) chelates. Acc Chem Res. 1999;32:941–949.
-
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KCP. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. - PubMed
-
- Tiefenauer LX, Kühne G, Andres RY. Antibody-magnetite nanoparticles: in vitro characterization of a potential tumor-specific contrast agent for magnetic resonance imaging. Bioconjugate Chem. 1993;4:347–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

