Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine
- PMID: 18234801
- PMCID: PMC2268460
- DOI: 10.1128/JVI.02244-07
Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine
Abstract
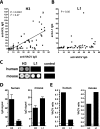
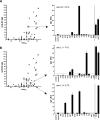
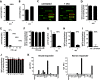
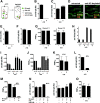
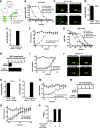
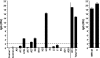
The smallpox vaccine is widely considered the gold standard for human vaccines, yet the key antibody targets in humans remain unclear. We endeavored to identify a stereotypic, dominant, mature virion (MV) neutralizing antibody target in humans which could be used as a diagnostic serological marker of protective humoral immunity induced by the smallpox vaccine (vaccinia virus [VACV]). We have instead found that diversity is a defining characteristic of the human antibody response to the smallpox vaccine. We show that H3 is the most immunodominant VACV neutralizing antibody target, as determined by correlation analysis of immunoglobulin G (IgG) specificities to MV neutralizing antibody titers. It was determined that purified human anti-H3 IgG is sufficient for neutralization of VACV; however, depletion or blockade of anti-H3 antibodies revealed no significant reduction in neutralization activity, showing anti-H3 IgG is not required in vaccinated humans (or mice) for neutralization of MV. Comparable results were obtained for human (and mouse) anti-L1 IgG and even for anti-H3 and anti-L1 IgG in combination. In addition to H3 and L1, human antibody responses to D8, A27, D13, and A14 exhibited statistically significant correlations with virus neutralization. Altogether, these data indicate the smallpox vaccine succeeds in generating strong neutralizing antibody responses not by eliciting a stereotypic response to a single key antigen but instead by driving development of neutralizing antibodies to multiple viral proteins, resulting in a "safety net" of highly redundant neutralizing antibody responses, the specificities of which can vary from individual to individual. We propose that this is a fundamental attribute of the smallpox vaccine.
Figures





References
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 796260-6271. - PMC - PubMed
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. L. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 34159-71. - PubMed
-
- Alibek, K. 27 March 1998. Russia's deadly expertise, p. A19. In The New York Times. The New York Times Co., New York, NY.
-
- Alibek, K., and S. Handelman. 1999. Biohazard. Random House, New York, NY.
-
- Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211320-337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

