Human HLA-DR transgenes protect mice from fatal virus-induced encephalomyelitis and chronic demyelination
- PMID: 18234804
- PMCID: PMC2268466
- DOI: 10.1128/JVI.02243-07
Human HLA-DR transgenes protect mice from fatal virus-induced encephalomyelitis and chronic demyelination
Abstract
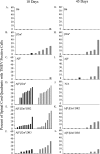
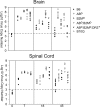
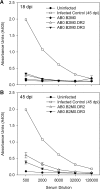
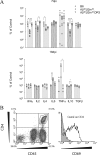
We evaluated the participatory role of human HLA-DR molecules in control of virus from the central nervous system and in the development of subsequent spinal cord demyelination. The experiments utilized intracranial infection with Theiler's murine encephalomyelitis virus (TMEV), a picornavirus that, in some strains of mice, results in primary demyelination. We studied DR2 and DR3 transgenic mice that were bred onto a combined class I-deficient mouse (beta-2 microglobulin deficient; beta2m(0)) and class II-deficient mouse (Abeta(0)) of the H-2(b) background. Abeta(0).beta2m(0) mice infected with TMEV died within 18 days of infection. These mice showed severe encephalomyelitis due to rapid replication of virus genome. In contrast, transgenic mice with insertion of a single human class II major histocompatibility complex (MHC) gene (DR2 or DR3) survived the acute infection. DR2 and DR3 mice controlled virus infection by 45 days and did not develop spinal cord demyelination. Levels of virus RNA were reduced in HLA-DR transgenic mice compared to Abeta(0).beta2m(0) mice. Virus-neutralizing antibody responses did not explain why DR mice survived the infection and controlled virus replication. However, DR mice showed an increase in gamma interferon and interleukin-2 transcripts in the brain, which were associated with protection. The findings support the hypothesis that the expression of a single human class II MHC molecule can, by itself, influence the control of an intracerebral pathogen in a host without a competent class I MHC immune response. The mechanism of protection appears to be the result of cytokines released by CD4(+) T cells.
Figures




Similar articles
-
Human class I major histocompatibility complex alleles determine central nervous system injury versus repair.J Neuroinflammation. 2016 Nov 17;13(1):293. doi: 10.1186/s12974-016-0759-4. J Neuroinflammation. 2016. PMID: 27855706 Free PMC article.
-
Transgenic expression of Theiler's murine encephalomyelitis virus genes in H-2(b) mice inhibits resistance to virus-induced demyelination.J Virol. 2002 Aug;76(15):7799-811. doi: 10.1128/jvi.76.15.7799-7811.2002. J Virol. 2002. PMID: 12097592 Free PMC article.
-
HLA-DQ polymorphism influences progression of demyelination and neurologic deficits in a viral model of multiple sclerosis.Mol Cell Neurosci. 2000 Jun;15(6):495-509. doi: 10.1006/mcne.2000.0843. Mol Cell Neurosci. 2000. PMID: 10860577 Free PMC article.
-
Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison.Microsc Res Tech. 1995 Oct 15;32(3):215-29. doi: 10.1002/jemt.1070320305. Microsc Res Tech. 1995. PMID: 8527856 Free PMC article. Review.
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
Cited by
-
Differential expression of multiple kallikreins in a viral model of multiple sclerosis points to unique roles in the innate and adaptive immune response.Biol Chem. 2014 Sep;395(9):1063-73. doi: 10.1515/hsz-2014-0141. Biol Chem. 2014. PMID: 25153387 Free PMC article.
-
Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis.Brain Pathol. 2012 Sep;22(5):709-22. doi: 10.1111/j.1750-3639.2012.00577.x. Epub 2012 Mar 21. Brain Pathol. 2012. PMID: 22335454 Free PMC article.
References
-
- Borson, N. D., C. Paul, X. Lin, W. K. Nevala, M. A. Strausbauch, M. Rodriguez, and P. J. Wettstein. 1997. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J. Virol. 715244-5250. - PMC - PubMed
-
- Drescher, K. M., L. T. Nguyen, V. Taneja, M. J. Coenen, J. L. Leibowitz, G. Strauss, G. J. Hammerling, C. S. David, and M. Rodriguez. 1998. Expression of the human histocompatibility leukocyte antigen DR3 transgene reduces the severity of demyelination in a murine model of multiple sclerosis. J. Clin. Investig. 1011765-1774. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

