Interfacial adsorption of antifreeze proteins: a neutron reflection study
- PMID: 18234809
- PMCID: PMC2480683
- DOI: 10.1529/biophysj.107.124560
Interfacial adsorption of antifreeze proteins: a neutron reflection study
Abstract
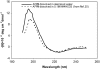
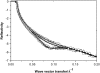
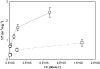
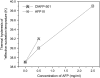
Interfacial adsorption from two antifreeze proteins (AFP) from ocean pout (Macrozoarces americanus, type III AFP, AFP III, or maAFP) and spruce budworm (Choristoneura fumiferana, isoform 501, or cfAFP) were studied by neutron reflection. Hydrophilic silicon oxide was used as model substrate to facilitate the solid/liquid interfacial measurement so that the structural features from AFP adsorption can be examined. All adsorbed layers from AFP III could be modeled into uniform layer distribution assuming that the protein molecules were adsorbed with their ice-binding surface in direct contact with the SiO(2) substrate. The layer thickness of 32 A was consistent with the height of the molecule in its crystalline form. With the concentration decreasing from 2 mg/ml to 0.01 mg/ml, the volume fraction of the protein packed in the monolayer decreased steadily from 0.4 to 0.1, consistent with the concentration-dependent inhibition of ice growth observed over the range. In comparison, insect cfAFP showed stronger adsorption over the same concentration range. Below 0.1 mg/ml, uniform layers were formed. But above 1 mg/ml, the adsorbed layers were characterized by a dense middle layer and two outer diffuse layers, with a total thickness around 100 A. The structural transition indicated the responsive changes of conformational orientation to increasing surface packing density. As the higher interfacial adsorption of cfAFP was strongly correlated with the greater thermal hysteresis of spruce budworm, our results indicated the important relation between protein adsorption and antifreeze activity.
Figures
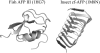


References
-
- Fletcher, G. L., C. L. Hew, and P. L. Davies. 2001. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 63:359–390. - PubMed
-
- Antson, A. A., D. J. Smith, D. I. Roper, S. Lewis, L. S. Caves, C. S. Verma, S. L. Buckley, P. J. Lillford, and R. E. Hubbard. 2001. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 305:875–889. - PubMed
-
- Sicheri, F., and D. S. C. Yang. 1995. Ice binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 375:427–431. - PubMed
-
- Duman, J. G. 2001. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 63:327–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

