Fluctuations of the red blood cell membrane: relation to mechanical properties and lack of ATP dependence
- PMID: 18234829
- PMCID: PMC2367166
- DOI: 10.1529/biophysj.107.117952
Fluctuations of the red blood cell membrane: relation to mechanical properties and lack of ATP dependence
Abstract
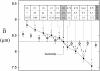
We have analyzed the fluctuations of the red blood cell membrane in both the temporal ((omega(s(-1))) and spatial (q(m(-1))) frequency domains. The cells were examined over a range of osmolarities leading to cell volumes from 50% to 170% of that in the isotonic state. The fluctuations of the isotonic cell showed an approximately q(-3)-dependence, indicative of a motion dominated by bending, with an inferred bending modulus of approximately 9 x 10(-19) J. When the cells were osmotically swollen to just below the point of lysis (166% of physiological volume), a q(-1)-dependence of the fluctuations supervened, implying that the motion was now dominated by membrane tension; estimated as approximately 1.3 x 10(-4) nm(-1). When, on the other hand, the cells were osmotically dehydrated, the fluctuation amplitude progressively decreased. This was caused by a rise in internal viscosity, as shown by measurements on resealed ghosts containing a reduced hemoglobin concentration, which displayed no such effect. We examined, in addition, cells depleted of ATP, before the onset of echinocytosis, and could observe no change in fluctuation amplitude. We conclude that the membrane fluctuations of the red cell are governed by bending modulus, membrane tension, and cytosolic viscosity, with little or no dependence on the presence or absence of ATP.
Figures





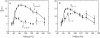

References
-
- Mohandas, N., and E. Evans. 1994. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu. Rev. Biophys. Biomol. Struct. 23:787–818. - PubMed
-
- Browicz, T. 1890. Further observation of motion phenomena on red blood cells in pathological states. Zbl. med. Wissen. 28:625–627.
-
- Parpart, A. K., and J. F. Hoffman. 1956. Flicker in erythrocytes; vibratory movements in the cytoplasm. J. Cell. Physiol. 47:295–303. - PubMed
-
- Brochard, F., and J. F. Lennon. 1975. Frequency spectrum of the flicker phenomenon in erythrocytes. J. Phys. 36:1035–1047.
-
- Lennon, J. F. 1977. The flicker of the erythrocyte. PhD thesis. Université Paris-Sud,: Orsay, France.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

