Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation
- PMID: 18234885
- PMCID: PMC6671418
- DOI: 10.1523/JNEUROSCI.1565-06.2008
Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation
Abstract
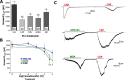
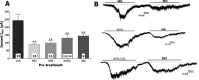
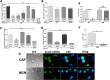
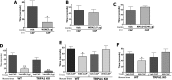
Although the cannabinoid agonists R-(+)-(2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrol[1,2,3-de]-1,4-benzoxazin-6-yl)-(1-naphthalenyl) methanone mesylate [WIN 55,212-2 (WIN)] and (R,S)-3-(2-iodo-5-nitrobenzoyl)-1-(1-methyl-2-piperidinylmethyl)-1H-indole (AM1241) exert peripheral antihyperalgesia in inflammatory pain models, the mechanism for cannabinoid-induced inhibition of nociceptive sensory neurons has not been fully studied. Because TRPV1 and TRPA1 channels play important roles in controlling hyperalgesia in inflammatory pain models, we investigated their modulation by WIN and AM1241. The applications of WIN (>5 microM) and AM1241 (>30 microM) inhibit responses of sensory neurons to capsaicin and mustard oil. To determine potential mechanisms for the inhibition, we evaluated cannabinoid effects on nociceptors. WIN and AM1241 excite sensory neurons in a concentration-dependent manner via a nonselective Ca2+-permeable channel. The expression of TRP channels in CHO cells demonstrates that both WIN and AM1241 activate TRPA1 and, by doing so, attenuate capsaicin and mustard oil responses. Using TRPA1-specific small interfering RNA or TRPA1-deficient mice, we show that the TRPA1 channel is a sole target through which WIN and mustard oil activate sensory neurons. In contrast, AM1241 activation of sensory neurons is mediated by TRPA1 and an unknown channel. The knockdown of TRPA1 activity in neurons completely eliminates the desensitizing effects of WIN and AM1241 on capsaicin-activated currents. Furthermore, the WIN- or AM1241-induced inhibition of capsaicin-evoked nocifensive behavior via peripheral actions is reversed in TRPA1 null-mutant mice. Together, this study demonstrates that certain cannabinoids exert their peripheral antinocifensive actions via activation of the TRPA1 channel on sensory neurons.
Figures




References
-
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
-
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. - PubMed
-
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
