The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration
- PMID: 18234889
- PMCID: PMC2800945
- DOI: 10.1523/JNEUROSCI.4853-07.2008
The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration
Abstract
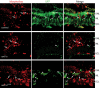
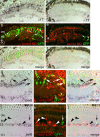
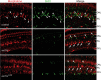
Unlike mammals, teleost fish can regenerate an injured retina, restoring lost visual function. Little is known of the molecular events that underlie retina regeneration. We previously found that in zebrafish, retinal injury stimulates Müller glia to generate multipotent alpha1-tubulin (alpha1T) and pax6-expressing progenitors for retinal repair. Here, we report the identification of a critical E-box in the alpha1T promoter that mediates transactivation by achaete-scute complex-like 1a (ascl1a) during retina regeneration. More importantly, we show that ascl1a is essential for retina regeneration. Within 4 h after retinal injury, ascl1a is induced in Müller glia. Knockdown of ascl1a blocks the induction of alpha1T and pax6 as well as Müller glial proliferation, consequently preventing the generation of retinal progenitors and their differentiated progeny. These data suggest ascl1a is required to convert quiescent Müller glia into actively dividing retinal progenitors, and that ascl1a is a key regulator in initiating retina regeneration.
Figures





References
-
- Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498. - PubMed
-
- Barthel LK, Raymond PA. In situ hybridization studies of retinal neurons. Methods Enzymol. 2000;316:579–590. - PubMed
-
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Develop Biol. 1998;9:591–597. - PubMed
-
- Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol Vis. 2005;11:775–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
