Complement-associated deposits in the human retina
- PMID: 18235023
- PMCID: PMC3947388
- DOI: 10.1167/iovs.07-1072
Complement-associated deposits in the human retina
Abstract
Purpose: The purpose of this study was to investigate complement activation and associated inflammatory mechanisms in normal, aged human retina.
Methods: Evidence of complement activation and associated mechanisms was assessed in normal human retina (n = 52) using a panel of antibodies directed against membrane attack complex (C5b-9), microglia (CD11b), amyloid precursor protein (APP), scavenger receptor (CD36), and a phytolectin (RCA-I). Fifty-two eyes, categorized into two age groups, were used. Nineteen "younger" eyes (<56 years) and 33 "older" eyes (>69 years) with no history of ocular disease were processed between 4 and 22 hours, with a median delay of 14 hours postmortem.
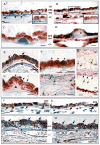
Results: Age-dependent expression was evident in C5b-9, APP, CD11b, and RCA-I, but not CD36, immunoreactivity. Immunoreactivity for C5b-9 was robust in Bruch membrane (BM) and the intercapillary pillars of Bruch. Immunoreactivity for APP was robust in the basal cytoplasm of the retinal pigment epithelium. Immunoreactivity for CD11b was robust on the surface of the retinal pigment epithelial cell, in the choriocapillaris, and in BM. Lectin binding of RCA-I was strong throughout the neuroretina.
Conclusions: Robust immunostaining for APP in older donor eyes suggested that amyloid beta peptides may be one of the triggers of complement activation during the normal aging process. Microglial markers CD11b and RCA-I also increase with age, suggesting a concomitant inflammatory response to C5b-9 deposits in the retinal pigment epithelium, BM, and CC. Immunoreactivity for CD36 was strong in both age groups; the lack of age dependence in this candidate receptor for amyloid beta suggested that complement activation may arise from interactions of amyloid beta with other candidate receptors in normal human retina.
Figures

References
-
- Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. - PubMed
-
- Edwards A, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. - PubMed
-
- Dentchev T, Milam AH, Lee VMY, Trojanowski JQ, Dunaief JL. Amyloid beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

