ATF3 protects against renal ischemia-reperfusion injury
- PMID: 18235102
- PMCID: PMC2396738
- DOI: 10.1681/ASN.2005111155
ATF3 protects against renal ischemia-reperfusion injury
Abstract
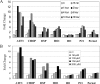
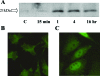
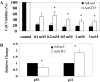
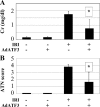
Oxidative stress-induced cell death plays a major role in the progression of ischemic acute renal failure. Using microarrays, we sought to identify a stress-induced gene that may be a therapeutic candidate. Human proximal tubule (HK2) cells were treated with hydrogen peroxide (H2O2) and RNA was applied to an Affymetrix gene chip. Five genes were markedly induced in a parallel time-dependent manner by cluster analysis, including activating transcription factor 3 (ATF3), p21(WAF1/CiP1) (p21), CHOP/GADD153, dual-specificity protein phosphatase, and heme oxygenase-1. H2O2 rapidly induced ATF3 approximately 12-fold in HK2 cells and approximately 6.5-fold in a mouse model of renal ischemia-reperfusion injury. Adenovirus-mediated expression of ATF3 protected HK2 cells against H2O2-induced cell death, and this was associated with a decrease of p53 mRNA and an increase of p21 mRNA. Moreover, when ATF3 was overexpressed in mice via adenovirus-mediated gene transfer, ischemia-reperfusion injury was reduced. In conclusion, ATF3 plays a protective role in renal ischemia-reperfusion injury and the mechanism of the protection may involve suppression of p53 and induction of p21.
Figures




References
-
- Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988–2002. J Am Soc Nephrol 17: 1143–1150, 2006 - PubMed
-
- Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 - PubMed
-
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT: Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128: 194–203, 1998 - PubMed
-
- Kazmers A, Jacobs L, Perkins A: The impact of complications after vascular surgery in Veterans Affairs Medical Centers. J Surg Res 67: 62–66, 1997 - PubMed
-
- Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ 3rd, Zentella A, Albert JD, Shires GT, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science 234: 470–474, 1986 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

