Gap junctions couple astrocytes and oligodendrocytes
- PMID: 18236012
- PMCID: PMC2650399
- DOI: 10.1007/s12031-007-9027-5
Gap junctions couple astrocytes and oligodendrocytes
Abstract
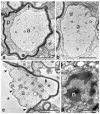
In vertebrates, a family of related proteins called connexins form gap junctions (GJs), which are intercellular channels. In the central nervous system (CNS), GJs couple oligodendrocytes and astrocytes (O/A junctions) and adjacent astrocytes (A/A junctions), but not adjacent oligodendrocytes, forming a "glial syncytium." Oligodendrocytes and astrocytes each express different connexins. Mutations of these connexin genes demonstrate that the proper functioning of myelin and oligodendrocytes requires the expression of these connexins. The physiological function of O/A and A/A junctions, however, remains to be illuminated.
Figures



References
-
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: The molecular and biophysical bases for the X-linked form of Charcot—Marie—Tooth disease. Brain Research Reviews. 2000;32:203–214. - PubMed
-
- Ahmad S, Chen SP, Sun JJ, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochemical and Biophysical Research Communications. 2003;307:362–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

