A33 antigen displays persistent surface expression
- PMID: 18236042
- PMCID: PMC2836164
- DOI: 10.1007/s00262-007-0433-x
A33 antigen displays persistent surface expression
Abstract
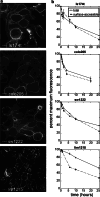
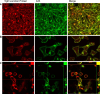
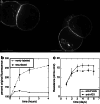
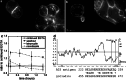
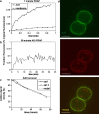
The A33 antigen is a cell surface glycoprotein of the small intestine and colonic epithelium with homology to tight junction-associated proteins of the immunoglobulin superfamily, including CAR and JAM. Its restricted tissue localization and high level of expression have led to its use as a target in colon cancer immunotherapy. Although the antigen is also present in normal intestine, radiolabeled antibodies against A33 are selectively retained by tumors in the gut as well as in metastatic lesions for as long as 6 weeks. Accordingly, we have studied the trafficking and kinetic properties of the antigen to determine its promise in two-step, pretargeted therapies. The localization, mobility, and persistence of the antigen were investigated, and this work has demonstrated that the antigen is both highly immobile and extremely persistent-retaining its surface localization for a turnover halflife of greater than 2 days. In order to explain these unusual properties, we explored the possibility that A33 is a component of the tight junction. The simple property of surface persistence, described here, may contribute to the prolonged retention of the clinically administered antibodies, and their uncommon ability to penetrate solid tumors.
Figures
References
-
- Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, Theodore LJ, Wilbur DS, Matthews DC, Press OW. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101(6):2340–2348. doi: 10.1182/blood-2002-03-0874. - DOI - PubMed
-
- Gruaz-Guyon A, Raguin O, Barbet J. Recent advances in pretargeted radioimmunotherapy. Curr Med Chem. 2005;12(3):319–338. - PubMed
-
- Boerman OC, van Schaijk FG, Oyen WJ, Corstens FH. Pretargeted radioimmunotherapy of cancer: progress step by step. J Nucl Med. 2003;44(3):400–411. - PubMed
-
- Johnstone CN, Tebbutt NC, Abud HE, White SJ, Stenvers KL, Hall NE, Cody SH, Whitehead RH, Catimel B, Nice EC, Burgess AW, Heath JK. Characterization of mouse A33 antigen, a definitive marker for basolateral surfaces of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;279(3):G500–G510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

