Betahistine in the treatment of vertiginous syndromes: a meta-analysis
- PMID: 18236637
- PMCID: PMC2640000
Betahistine in the treatment of vertiginous syndromes: a meta-analysis
Abstract
Vertigo is a very frequent disorder, associated with highly disabling symptomatology. Since the aetiology cannot always be easily identified, treatment is often addressed to the symptoms. Betahistine, a drug characterized by a multi-factorial mode of action of the modulatory type, has been widely employed in the management of various vertiginous syndromes. Its use in Italy is, currently, authorized to treat the vertiginous symptoms related to Ménière's disease. A meta-analysis has, therefore, been carried out to assess, the efficacy of betahistine in the treatment of other vertiginous syndromes, such as positional paroxysmal vertigo (cupulo-canalolithiasis) and vertigo secondary to arterial deficiency of the vertebrobasilar area, regardless of the specific cause. A review has been made of the literature concerning clinical trials performed with betahistine versus placebo in a randomised double-blind, parallel-group or cross-over design. Only studies evaluating betahistine in patients with vertiginous symptomatology not related to Ménière's disease were selected. Of the 104 publications, obtained from an analysis of "Medline", "EMBASE" and "CINAHL" databases, 7 clinical studies, which met the selection criteria, for a total of 367 patients, were extrapolated and analysed. The meta-analysis was conducted using the "Cochrane Collaboration's Review Manager" software in all the case series and in the sub-groups identified by the experimental design (parallel or crossover design), range of dosages (32-48 mg/day) and range of treatment duration (from 3 weeks to 4 months). The various parameters used to evaluate efficacy, adopted in the trials, and taken into account in the metaanalysis, as overall judgement of the patient or physician, number of vertiginous episodes and their duration, were classified according to the binary classification of "improved" and "not improved". The results of the meta-analysis confirm the therapeutic benefit of betahistine versus placebo. In particular, the investigation carried out on the overall sample shows an odds ratio of 3.52 (95% confidence interval 2.40-5.18) and a relative risk of 1.78 (95% confidence interval 1.48-2.13), while the analysis of the sub-groups denotes a maximum efficacy after doses of 32 to 36 mg and with a period of treatment of 3-8 weeks. The present meta-analysis confirms the benefit of drug treatment with betahistine for the vertiginous symptomatology related to cupulo-canalolithiasis and vertebro-basilar arterial insufficiency.
La vertigine è un disturbo molto frequente, associato ad una sintomatologia fortemente invalidante. Poiché la sua eziologia non risulta sempre di facile identificazione, le terapie impiegate sono spesso indirizzate al trattamento dei sintomi. La betaistina, molecola caratterizzata da un meccanismo d’azione multifattoriale di tipo modulatorio, ha trovato un vasto impiego nel trattamento delle sindromi vertiginose di varia natura. Attualmente in Italia il suo impiego è autorizzato per il trattamento della sintomatologia vertiginosa correlata alla malattia di Ménière. Si è voluto quindi valutare, attraverso una meta-analisi, l’efficacia di betaistina nel trattamento di altre sindromi vertiginose, quali la vertigine parossistica di posizione (cupolo–canalolitiasi) e la vertigine secondaria a deficit arterioso, qualunque ne sia la causa specifica, del distretto vertebro-basilare. È stata considerata la letteratura riguardante studi clinici controllati, randomizzati a gruppi paralleli o crossover, condotti in doppio cieco, betaistina vs. placebo. Sono stati selezionati i soli studi volti alla valutazione di betaistina in pazienti con sintomatologia vertiginosa non riferibile a malattia di Ménière. Dalle 104 pubblicazioni estrapolate mediante analisi dei database Medline, EMBASE e CINAHL, sono stati estratti ed analizzati 7 studi clinici che rispondevano ai criteri scelti, per un totale di 367 pazienti. La meta-analisi è stata condotta mediante l’utilizzo del Cochrane Collaboration’s Review Manager software sia sull’intera casistica, sia su sottogruppi identificati dal disegno sperimentale (parallelo o crossover), dai range di dosaggi (da 32 a 48 mg/die) e dai range di durata del trattamento (da 1 a 3 mesi). I diversi parametri di valutazione dell’efficacia usati nei trial e presi in considerazione nella meta-analisi, quali il giudizio complessivo del paziente o del medico, il numero di episodi vertiginosi e la loro durata, sono stati uniformati secondo la classificazione binaria di “Migliorati” e “Non migliorati”. I risultati della meta-analisi confermano il beneficio terapeutico di betaistina vs. placebo. In particolare, l’indagine condotta sul campione totale evidenzia un Odds Ratio (OR) di 3,52 (IC 95% 2,40-5,18) ed un Relative Risk (RR) di 1,78 (IC 95% 1,48-2,13), mentre l’analisi dei sottogruppi suggerisce un’efficacia massima ottenuta a dosaggi compresi tra 32 e 36 mg e con periodo di trattamento di 3-8 settimane. La presente meta-analisi conferma l’utilità del trattamento farmacologico con betaistina della sintomatologia vertiginosa correlata alla cupolo-canalolitiasi ed alla insufficienza arteriosa vertebro-basilare.
Figures





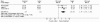

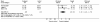
References
-
- Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo. Elucidation of mechanisms of action. CNS Drugs 2001;15:853-70. - PubMed
-
- Arrang JM, Garbarg M, Quach TT, Tuong MDT, Yeramian E, Schwartz JC. Actions of betahistine at histamine receptors in the brain. Eur J Pharm 1985;111:73-84. - PubMed
-
- Wilmot TJ, Menon GN. Betahistine in Ménière’s disease. J Laryngol Otol 1976;9:833-40. - PubMed
-
- The mechanism of action of betahistine: towards a consensus. IMN (International Medical News) 1999;99:1-6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
