Functional role of the additional domains in inulosucrase (IslA) from Leuconostoc citreum CW28
- PMID: 18237396
- PMCID: PMC2270844
- DOI: 10.1186/1471-2091-9-6
Functional role of the additional domains in inulosucrase (IslA) from Leuconostoc citreum CW28
Abstract
Background: Inulosucrase (IslA) from Leuconostoc citreum CW28 belongs to a new subfamily of multidomain fructosyltransferases (FTFs), containing additional domains from glucosyltransferases. It is not known what the function of the additional domains in this subfamily is.
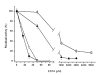
Results: Through construction of truncated versions we demonstrate that the acquired regions are involved in anchoring IslA to the cell wall; they also confer stability to the enzyme, generating a larger structure that affects its kinetic properties and reaction specificity, particularly the hydrolysis and transglycosylase ratio. The accessibility of larger molecules such as EDTA to the catalytic domain (where a Ca2+ binding site is located) is also affected as demonstrated by the requirement of 100 times higher EDTA concentrations to inactivate IslA with respect to the smallest truncated form.
Conclusion: The C-terminal domain may have been acquired to anchor inulosucrase to the cell surface. Furthermore, the acquired domains in IslA interact with the catalytic core resulting in a new conformation that renders the enzyme more stable and switch the specificity from a hydrolytic to a transglycosylase mechanism. Based on these results, chimeric constructions may become a strategy to stabilize and modulate biocatalysts based on FTF activity.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

