Transcriptional activation of the suppressor of cytokine signaling-3 (SOCS-3) gene via STAT3 is increased in F9 REX1 (ZFP-42) knockout teratocarcinoma stem cells relative to wild-type cells
- PMID: 18237746
- PMCID: PMC2972193
- DOI: 10.1016/j.jmb.2007.12.038
Transcriptional activation of the suppressor of cytokine signaling-3 (SOCS-3) gene via STAT3 is increased in F9 REX1 (ZFP-42) knockout teratocarcinoma stem cells relative to wild-type cells
Abstract
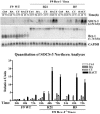
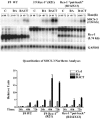
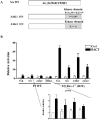
Rex1 (Zfp42), first identified as a gene that is transcriptionally repressed by retinoic acid (RA), encodes a zinc finger transcription factor expressed at high levels in F9 teratocarcinoma stem cells, embryonic stem cells, and other stem cells. Loss of both alleles of Rex1 by homologous recombination alters the RA-induced differentiation of F9 cells, a model of pluripotent embryonic stem cells. We identified Suppressor of Cytokine Signaling-3 (SOCS-3) as a gene that exhibits greatly increased transcriptional activation in RA, cAMP, and theophylline (RACT)-treated F9 Rex1(-/-) cells (approximately 25-fold) as compared to wild-type (WT) cells ( approximately 2.5-fold). By promoter deletion, mutation, and transient transfection analyses, we have shown that this transcriptional increase is mediated by the STAT3 DNA-binding elements located between -99 to -60 in the SOCS-3 promoter. Overexpression of STAT3 dominant-negative mutants greatly diminishes this SOCS-3 transcriptional increase in F9 Rex1(-/-) cells. This increase in SOCS-3 transcription is associated with a four- to fivefold higher level of tyrosine-phosphorylated STAT3 in the RACT-treated F9 Rex1(-/-) cells as compared to WT. Dominant-negative Src tyrosine kinase, Jak2, and protein kinase A partially reduce the transcriptional activation of the SOCS 3 gene in RACT-treated F9 Rex1 null cells. In contrast, parathyroid hormone peptide enhances the effect of RA in F9 Rex1(-/-) cells, but not in F9 WT. Thus, Rex1, which is highly expressed in stem cells, inhibits signaling via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, thereby modulating the differentiation of F9 cells.
Figures









References
-
- Gudas LJ. Molecular mechanisms of retinoid action. Am J Respir Cell Mol Biol. 1990;2:319–20. - PubMed
-
- Gudas LJ. Retinoids and vertebrate development. J Biol Chem. 1994;269:15399–402. - PubMed
-
- Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Ann. Rev. Biochem. 1995;64:210–233. - PubMed
-
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007 In press. - PubMed
-
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

