Rosiglitazone increases fatty acid oxidation and fatty acid translocase (FAT/CD36) but not carnitine palmitoyltransferase I in rat muscle mitochondria
- PMID: 18238811
- PMCID: PMC2375706
- DOI: 10.1113/jphysiol.2007.146563
Rosiglitazone increases fatty acid oxidation and fatty acid translocase (FAT/CD36) but not carnitine palmitoyltransferase I in rat muscle mitochondria
Abstract
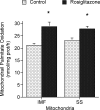
Peroxisome proliferator-activated receptors (PPARs) alter the expression of genes involved in regulating lipid metabolism. Rosiglitazone, a PPARgamma agonist, induces tissue-specific effects on lipid metabolism; however, its mode of action in skeletal muscle remains unclear. Since fatty acid translocase (FAT/CD36) was recently identified as a possible regulator of skeletal muscle fatty acid transport and mitochondrial fatty acid oxidation, we examined in this tissue the effects of rosiglitazone infusion (7 days, 1 mg day(-1)) on FAT/CD36 mRNA and protein, its plasmalemmal content and fatty acid transport. In addition, in isolated subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria we examined rates of fatty acid oxidation, FAT/CD36 and carnitine palmitoyltransferase I (CPTI) protein, and CPTI and beta-hydroxyacyl CoA dehydrogenase (beta-HAD) activities. Rosiglitazone did not alter FAT/CD36 mRNA or protein expression, FAT/CD36 plasmalemmal content, or the rate of fatty acid transport into muscle (P > 0.05). In contrast, rosiglitazone increased the rates of fatty acid oxidation in both SS (+21%) and IMF mitochondria (+36%). This was accompanied by concomitant increases in FAT/CD36 in subsarcolemmal (SS) (+43%) and intermyofibrillar (IMF) mitochondria (+46%), while SS and IMF CPTI protein content, and CPTI submaximal and maximal activities (P > 0.05) were not altered. Similarly, citrate synthase (CS) and beta-HAD activities were also not altered by rosiglitazone in SS and IMF mitochondria (P > 0.05). These studies provide another example whereby changes in mitochondrial fatty oxidation are associated with concomitant changes in mitochondrial FAT/CD36 independent of any changes in CPTI. Moreover, these studies identify for the first time a mechanism by which rosiglitazone stimulates fatty acid oxidation in skeletal muscle, namely the chronic, subcellular relocation of FAT/CD36 to mitochondria.
Figures

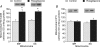

References
-
- Abumrad NA, El-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. - PubMed
-
- Albrektsen T, Frederiksen KS, Holmes WE, Boel E, Taylor K, Fleckner J. Novel genes regulated by the insulin sensitizer rosiglitazone during adipocyte differentiation. Diabetes. 2002;51:1042–1051. - PubMed
-
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. - PubMed
-
- Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Commun. 2004;323:249–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

