Dicer-dependent pathways regulate chondrocyte proliferation and differentiation
- PMID: 18238902
- PMCID: PMC2538863
- DOI: 10.1073/pnas.0707900105
Dicer-dependent pathways regulate chondrocyte proliferation and differentiation
Abstract
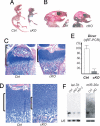
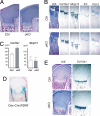
Small noncoding RNAs, microRNAs (miRNAs), bind to messenger RNAs through base pairing to suppress gene expression. Despite accumulating evidence that miRNAs play critical roles in various biological processes across diverse organisms, their roles in mammalian skeletal development have not been demonstrated. Here, we show that Dicer, an essential component for biogenesis of miRNAs, is essential for normal skeletal development. Dicer-null growth plates show a progressive reduction in the proliferating pool of chondrocytes, leading to severe skeletal growth defects and premature death of mice. The reduction of proliferating chondrocytes in Dicer-null growth plates is caused by two distinct mechanisms: decreased chondrocyte proliferation and accelerated differentiation into postmitotic hypertrophic chondrocytes. These defects appear to be caused by mechanisms downstream or independent of the Ihh-PTHrP signaling pathway, a pivotal signaling system that regulates chondrocyte proliferation and differentiation. Microarray analysis of Dicer-null chondrocytes showed limited expression changes in miRNA-target genes, suggesting that, in the majority of cases, chondrocytic miRNAs do not directly regulate target RNA abundance. Our results demonstrate the critical role of the Dicer-dependent pathway in the regulation of chondrocyte proliferation and differentiation during skeletal development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Science. 2007;316:604–608. - PubMed
-
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Science. 2007;316:575–579. - PubMed
-
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Cell. 2007;129:303–317. - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Nat Genet. 2003;35:215–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

