Dimethylarginine dimethylaminohydrolase overexpression enhances insulin sensitivity
- PMID: 18239148
- PMCID: PMC3165027
- DOI: 10.1161/ATVBAHA.108.162073
Dimethylarginine dimethylaminohydrolase overexpression enhances insulin sensitivity
Abstract
Objective: Previous studies suggest that nitric oxide (NO) may modulate insulin-induced uptake of glucose in insulin-sensitive tissues. Asymmetrical dimethylarginine (ADMA) is an endogenous inhibitor of NO synthase (NOS). We hypothesized that a reduction in endogenous ADMA would increase NO synthesis and thereby enhance insulin sensitivity.
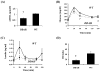
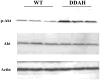
Methods and results: To test this hypothesis we used a transgenic mouse in which we overexpressed human dimethylarginine dimethylaminohydrolase (DDAH-I). The DDAH-I mice had lower plasma ADMA at all ages (22 to 70 wk) by comparison to wild-type (WT) littermates. With a glucose challenge, WT mice showed a prompt increase in ADMA, whereas DDAH-I mice had a blunted response. Furthermore, DDAH-I mice had a blunted increase in plasma insulin and glucose levels after glucose challenge, with a 50% reduction in the insulin resistance index, consistent with enhanced sensitivity to insulin. In liver, we observed an increased Akt phosphorylation in the DDAH-I mice after i.p. glucose challenge. Incubation of skeletal muscle from WT mice ex vivo with ADMA (2 mumol/L) markedly suppressed insulin-induced glycogen synthesis in fast-twitch but not slow-twitch muscle.
Conclusions: These findings suggest that the endogenous NOS inhibitor ADMA reduces insulin sensitivity, consistent with previous observations that NO plays a role in insulin sensitivity.
Figures



References
-
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. - PubMed
-
- Reaven G. Insulin resistance, type 2 diabetes mellitus, and cardiovascular disease: the end of the beginning. Circulation. 2005;112:3030–3032. - PubMed
-
- Cooke JP. The endothelium: a new target for therapy. Vasc Med. 2000;5:49–53. - PubMed
-
- Baron AD. Vascular reactivity. Am J Cardiol. 1999;84:25J–27J. - PubMed
-
- Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49:684–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

