Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice
- PMID: 18239191
- PMCID: PMC2440259
- DOI: 10.1165/rcmb.2007-0342OC
Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice
Abstract
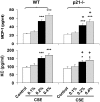
The cyclin-dependent kinase inhibitor p21(CIP1/WAF1/SDI1) (p21) is an important inhibitory checkpoint regulator of cell cycle progression in response to oxidative and genotoxic stresses. It is known that p21 potentiates inflammatory response and inhibits apoptosis and proliferation, leading to cellular senescence. However, the role of endogenous p21 in regulation of lung inflammatory and injurious responses by cigarette smoke (CS) or other pro-inflammatory stimuli is not known. We hypothesized that p21 is an important modifier of lung inflammation and injury, and genetic ablation of p21 will confer protection against CS and other pro-inflammatory stimuli (lipopolysacchride [LPS] and N-formyl-methionyl-leucyl-phenylalanine [fMLP])-mediated lung inflammation and injury. To test this hypothesis, p21-deficient (p21-/-) and wild-type mice were exposed to CS, LPS, or fMLP, and the lung oxidative stress and inflammatory responses as well as airspace enlargement were assessed. We found that targeted disruption of p21 attenuated CS-, LPS-, or fMLP-mediated lung inflammatory responses in mice. CS-mediated oxidative stress and fMLP-induced airspace enlargement were also decreased in lungs of p21-/- mice compared with wild-type mice. The mechanism underlying this finding was associated with decreased NF-kappaB activation, and reactive oxygen species generation by decreased phosphorylation of p47(phox) and down-modulating the activation of p21-activated kinase. Our data provide insight into the mechanism of pro-inflammatory effect of p21, and the loss of p21 protects against lung oxidative and inflammatory responses, and airspace enlargement in response to multiple pro-inflammatory stimuli. These data may have ramifications in CS-induced senescence in the pathogenesis of chronic obstructive pulmonary disease/emphysema.
Figures






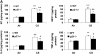
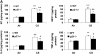
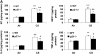




References
-
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. - PubMed
-
- Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:176–183. - PubMed
-
- Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 2007;170:855–864. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

