Regulation of an inducible promoter by an HP1beta-HP1gamma switch
- PMID: 18239689
- PMCID: PMC2267390
- DOI: 10.1038/embor.2008.1
Regulation of an inducible promoter by an HP1beta-HP1gamma switch
Abstract
The mammalian heterochromatin protein 1 (HP1) family of proteins was recently shown to be involved in transient repression of inducible promoters. One of these promoters is the HIV1 long terminal repeat, which, during viral latency, recruits a non-processive RNA polymerase II (RNAPII) that synthesizes a short regulatory transcript. Here, we have used this promoter to examine the interplay of HP1alpha, HP1beta and HP1gamma with RNAPII. We find that, in the absence of stimulation, HP1beta is present on the promoter together with the non-processive RNAPII and functions as a negative regulator. On activation, HP1beta bound to methylated H3K9 is rapidly released concurrent with histone H3 phospho-acetylation, and is replaced by HP1gamma. This isoform localizes to the promoter but also inside the coding region, together with the processive RNAPII. Our data show that HP1 recruitment-release is a sequential mechanism that is precisely regulated and highly dependent on transcription.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
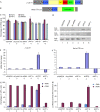
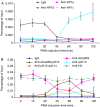
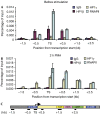

References
-
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS (2003) A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature 423: 659–663 - PubMed
-
- Batsché E, Yaniv M, Muchardt C (2006) The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol 13: 22–29 - PubMed
-
- Clayton AL, Mahadevan LC (2003) MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett 546: 51–58 - PubMed
-
- Daujat S, Zeissler U, Waldmann T, Happel N, Schneider R (2005) HP1 binds specifically to Lys 26-methylated histone H1.4, whereas simultaneous Ser 27 phosphorylation blocks HP1 binding. J Biol Chem 280: 38090–38095 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

